Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells
- PMID: 20374430
- PMCID: PMC2912144
- DOI: 10.1111/j.1471-4159.2010.06731.x
Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells
Abstract
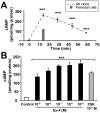
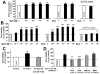
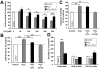
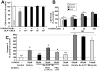
Increasing evidence suggests that glucagon-like peptide-1 (GLP-1), an incretin hormone of current interest in type 2 diabetes, is neuroprotective in both cell culture and animal models. To characterize the neuroprotective properties of GLP-1 and associated underlying mechanisms, we over-expressed the GLP-1 receptor (GLP-1R) on human neuroblastoma SH-SY5Y cells to generate a neuronal culture system featuring enhanced GLP-1R signaling. In GLP-1R over-expressing SH-SY5Y (SH-hGLP-1R#9) cells, GLP-1 and the long-acting agonist exendin-4 stimulated cell proliferation and increased cell viability by 2-fold at 24 h at physiologically relevant concentrations. This GLP-1R-dependent action was mediated via the protein kinase A and phosphoinositide 3-kinase signaling pathways, with the MAPK pathway playing a minor role. GLP-1 and exendin-4 pretreatment dose-dependently protected SH-hGLP-1R#9 cells from hydrogen peroxide (H(2)O(2))- and 6-hydroxydopamine-induced cell death. This involved amelioration of elevated caspase 3 activity, down-regulation of pro-apoptotic Bax and up-regulation of anti-apoptotic Bcl-2 protein. In the presence of 6-hydroxydopamine, GLP-1's ability to lower caspase-3 activity was abolished with the phosphoinositide 3-kinase inhibitor, LY2940002, and partly reduced with the protein kinase A inhibitor, H89. Hence, GLP-1R mediated neurotrophic and anti-apoptotic actions co-contribute to the neuroprotective property of GLP-1 in neuronal cell cultures, and reinforce the potential therapeutic value of GLP-1R agonists in neurodegenerative disorders involving oxidative stress.
Figures

Similar articles
-
The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells.Breast Cancer Res Treat. 2012 Apr;132(2):449-61. doi: 10.1007/s10549-011-1585-0. Epub 2011 Jun 3. Breast Cancer Res Treat. 2012. PMID: 21638053
-
Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice.J Neurochem. 2015 Dec;135(6):1203-1217. doi: 10.1111/jnc.13169. Epub 2015 Jun 18. J Neurochem. 2015. PMID: 25982185 Free PMC article.
-
Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A.Arterioscler Thromb Vasc Biol. 2010 Jul;30(7):1407-14. doi: 10.1161/ATVBAHA.110.206425. Epub 2010 May 6. Arterioscler Thromb Vasc Biol. 2010. PMID: 20448207
-
Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials.BMJ. 2012 Jan 10;344:d7771. doi: 10.1136/bmj.d7771. BMJ. 2012. PMID: 22236411 Free PMC article. Review.
-
A New Treatment Strategy for Parkinson's Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway.Cell Transplant. 2017 Sep;26(9):1560-1571. doi: 10.1177/0963689717721234. Cell Transplant. 2017. PMID: 29113464 Free PMC article. Review.
Cited by
-
Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment.Pharmacol Res. 2022 Dec;186:106550. doi: 10.1016/j.phrs.2022.106550. Epub 2022 Nov 11. Pharmacol Res. 2022. PMID: 36372278 Free PMC article. Review.
-
PT320, a Sustained-Release GLP-1 Receptor Agonist, Ameliorates L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson's Disease.Int J Mol Sci. 2023 Feb 28;24(5):4687. doi: 10.3390/ijms24054687. Int J Mol Sci. 2023. PMID: 36902115 Free PMC article.
-
A synopsis on the role of tyrosine hydroxylase in Parkinson's disease.CNS Neurol Disord Drug Targets. 2012 Jun 1;11(4):395-409. doi: 10.2174/187152712800792785. CNS Neurol Disord Drug Targets. 2012. PMID: 22483313 Free PMC article. Review.
-
The neuroprotective effects of glucagon-like peptide 1 in Alzheimer's and Parkinson's disease: An in-depth review.Front Neurosci. 2022 Sep 1;16:970925. doi: 10.3389/fnins.2022.970925. eCollection 2022. Front Neurosci. 2022. PMID: 36117625 Free PMC article. Review.
-
Echinometra lucunter molecules reduce Aβ42-induced neurotoxicity in SH-SY5Y neuron-like cells: effects on disaggregation and oxidative stress.J Venom Anim Toxins Incl Trop Dis. 2023 Dec 1;29:e20230031. doi: 10.1590/1678-9199-JVATITD-2023-0031. eCollection 2023. J Venom Anim Toxins Incl Trop Dis. 2023. PMID: 38053575 Free PMC article.
References
-
- Albani D, Polito L, Batelli S, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110:1445–1456. - PubMed
-
- Banks WA, Goulet M, Rusche JR, Niehoff ML, Boismenu R. Differential transport of a secretin analog across the blood-brain and blood-cerebrospinal fluid barriers of the mouse. J Pharmacol Exp Ther. 2002;302:1062–1069. - PubMed
-
- Banks WA, Uchida D, Arimura A, Somogyvári-Vigh A, Shioda S. Transport of pituitary adenylate cyclase-activating polypeptide across the blood-brain barrier and the prevention of ischemia-induced death of hippocampal neurons. Ann N Y Acad Sci. 1996;805:270–277. - PubMed
-
- Baggio L, Drucker D. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. - PubMed
-
- Bertilsson G, Patrone C, Zachrisson O, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86:326–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

