Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology
- PMID: 20368620
- PMCID: PMC2854367
- DOI: 10.1083/jcb.201001125
Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology
Abstract
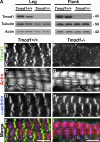
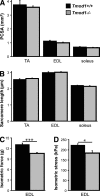
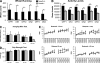
During myofibril assembly, thin filament lengths are precisely specified to optimize skeletal muscle function. Tropomodulins (Tmods) are capping proteins that specify thin filament lengths by controlling actin dynamics at pointed ends. In this study, we use a genetic targeting approach to explore the effects of deleting Tmod1 from skeletal muscle. Myofibril assembly, skeletal muscle structure, and thin filament lengths are normal in the absence of Tmod1. Tmod4 localizes to thin filament pointed ends in Tmod1-null embryonic muscle, whereas both Tmod3 and -4 localize to pointed ends in Tmod1-null adult muscle. Substitution by Tmod3 and -4 occurs despite their weaker interactions with striated muscle tropomyosins. However, the absence of Tmod1 results in depressed isometric stress production during muscle contraction, systemic locomotor deficits, and a shift to a faster fiber type distribution. Thus, Tmod3 and -4 compensate for the absence of Tmod1 structurally but not functionally. We conclude that Tmod1 is a novel regulator of skeletal muscle physiology.
Figures




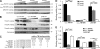
Similar articles
-
Tropomodulin 1 directly controls thin filament length in both wild-type and tropomodulin 4-deficient skeletal muscle.Development. 2015 Dec 15;142(24):4351-62. doi: 10.1242/dev.129171. Epub 2015 Nov 19. Development. 2015. PMID: 26586224 Free PMC article.
-
Functional effects of mutations in the tropomyosin-binding sites of tropomodulin1 and tropomodulin3.Cytoskeleton (Hoboken). 2014 Jul;71(7):395-411. doi: 10.1002/cm.21179. Epub 2014 Jul 2. Cytoskeleton (Hoboken). 2014. PMID: 24922351 Free PMC article.
-
Mammalian tropomodulins nucleate actin polymerization via their actin monomer binding and filament pointed end-capping activities.J Biol Chem. 2010 Oct 22;285(43):33265-33280. doi: 10.1074/jbc.M110.144873. Epub 2010 Jul 21. J Biol Chem. 2010. PMID: 20650902 Free PMC article.
-
Tropomodulin capping of actin filaments in striated muscle development and physiology.J Biomed Biotechnol. 2011;2011:103069. doi: 10.1155/2011/103069. Epub 2011 Oct 17. J Biomed Biotechnol. 2011. PMID: 22013379 Free PMC article. Review.
-
Capping actin filament growth: tropomodulin in muscle and nonmuscle cells.Soc Gen Physiol Ser. 1997;52:79-89. Soc Gen Physiol Ser. 1997. PMID: 9210222 Review.
Cited by
-
Tropomodulins and tropomyosins: working as a team.J Muscle Res Cell Motil. 2013 Aug;34(3-4):247-60. doi: 10.1007/s10974-013-9349-6. Epub 2013 Jul 5. J Muscle Res Cell Motil. 2013. PMID: 23828180 Free PMC article. Review.
-
Lens ion homeostasis relies on the assembly and/or stability of large connexin 46 gap junction plaques on the broad sides of differentiating fiber cells.Am J Physiol Cell Physiol. 2015 May 15;308(10):C835-47. doi: 10.1152/ajpcell.00372.2014. Epub 2015 Mar 4. Am J Physiol Cell Physiol. 2015. PMID: 25740157 Free PMC article.
-
Dynamic regulation of sarcomeric actin filaments in striated muscle.Cytoskeleton (Hoboken). 2010 Nov;67(11):677-92. doi: 10.1002/cm.20476. Cytoskeleton (Hoboken). 2010. PMID: 20737540 Free PMC article. Review.
-
Tropomodulin 1 constrains fiber cell geometry during elongation and maturation in the lens cortex.J Histochem Cytochem. 2012 Jun;60(6):414-27. doi: 10.1369/0022155412440881. Epub 2012 Apr 3. J Histochem Cytochem. 2012. PMID: 22473940 Free PMC article.
-
Congenital myopathy-causing tropomyosin mutations induce thin filament dysfunction via distinct physiological mechanisms.Hum Mol Genet. 2012 Oct 15;21(20):4473-85. doi: 10.1093/hmg/dds289. Epub 2012 Jul 13. Hum Mol Genet. 2012. PMID: 22798622 Free PMC article.
References
-
- Akkari P.A., Song Y., Hitchcock-DeGregori S., Blechynden L., Laing N. 2002. Expression and biological activity of Baculovirus generated wild-type human slow alpha tropomyosin and the Met9Arg mutant responsible for a dominant form of nemaline myopathy. Biochem. Biophys. Res. Commun. 296:300–304 10.1016/S0006-291X(02)00852-5 - DOI - PubMed
-
- Almenar-Queralt A., Gregorio C.C., Fowler V.M. 1999a. Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 112:1111–1123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

