Genetic demonstration of a redundant role of extracellular signal-regulated kinase 1 (ERK1) and ERK2 mitogen-activated protein kinases in promoting fibroblast proliferation
- PMID: 20368360
- PMCID: PMC2876689
- DOI: 10.1128/MCB.00131-10
Genetic demonstration of a redundant role of extracellular signal-regulated kinase 1 (ERK1) and ERK2 mitogen-activated protein kinases in promoting fibroblast proliferation
Abstract
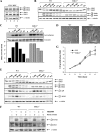
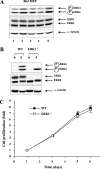
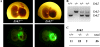
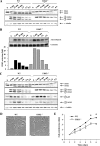
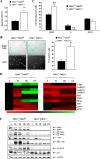
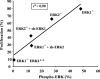
The extracellular signal-regulated kinase 1 and 2 (ERK1/2) mitogen-activated protein (MAP) kinase signaling pathway plays an important role in the proliferative response of mammalian cells to mitogens. However, the individual contribution of the isoforms ERK1 and ERK2 to cell proliferation control is unclear. The two ERK isoforms have similar biochemical properties and recognize the same primary sequence determinants on substrates. On the other hand, analysis of mice lacking individual ERK genes suggests that ERK1 and ERK2 may have evolved unique functions. In this study, we used a robust genetic approach to analyze the individual functions of ERK1 and ERK2 in cell proliferation using genetically matched primary embryonic fibroblasts. We show that individual loss of either ERK1 or ERK2 slows down the proliferation rate of fibroblasts to an extent reflecting the expression level of the kinase. Moreover, RNA interference-mediated silencing of ERK1 or ERK2 expression in cells genetically disrupted for the other isoform similarly reduces cell proliferation. We generated fibroblasts genetically deficient in both Erk1 and Erk2. Combined loss of ERK1 and ERK2 resulted in a complete arrest of cell proliferation associated with G(1) arrest and premature replicative senescence. Together, our findings provide compelling genetic evidence for a redundant role of ERK1 and ERK2 in promoting cell proliferation.
Figures

Similar articles
-
Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence.Cell Signal. 2013 Dec;25(12):2540-7. doi: 10.1016/j.cellsig.2013.08.014. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993963
-
ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially.J Biol. 2006;5(5):14. doi: 10.1186/jbiol38. J Biol. 2006. PMID: 16805921 Free PMC article.
-
Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels.Mol Cell Biol. 2008 Jan;28(1):511-27. doi: 10.1128/MCB.00800-07. Epub 2007 Oct 29. Mol Cell Biol. 2008. PMID: 17967895 Free PMC article.
-
The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition.Oncogene. 2007 May 14;26(22):3227-39. doi: 10.1038/sj.onc.1210414. Oncogene. 2007. PMID: 17496918 Review.
-
Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy.Ann N Y Acad Sci. 2010 Feb;1188:96-102. doi: 10.1111/j.1749-6632.2009.05088.x. Ann N Y Acad Sci. 2010. PMID: 20201891 Free PMC article. Review.
Cited by
-
Targeting the multifaceted BRAF in cancer: New directions.Oncotarget. 2024 Jul 16;15:486-492. doi: 10.18632/oncotarget.28612. Oncotarget. 2024. PMID: 39018217 Free PMC article. Review.
-
The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives.Biomimetics (Basel). 2024 Apr 12;9(4):230. doi: 10.3390/biomimetics9040230. Biomimetics (Basel). 2024. PMID: 38667241 Free PMC article. Review.
-
Cross Talk Between Cells and the Current Bioceramics in Bone Regeneration: A Comprehensive Review.Cell Transplant. 2024 Jan-Dec;33:9636897241236030. doi: 10.1177/09636897241236030. Cell Transplant. 2024. PMID: 38494898 Free PMC article. Review.
-
Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets.Signal Transduct Target Ther. 2023 Jul 31;8(1):282. doi: 10.1038/s41392-023-01501-9. Signal Transduct Target Ther. 2023. PMID: 37518181 Free PMC article. Review.
-
Mechanism of Key Ingredient of Astragalus membranaceus on Lung Adenocarcinoma via PI3K/AKT Signaling Clarified by Utilizing Network Pharmacology Approach and Experimental Validation.Chin J Integr Med. 2023 Mar;29(3):244-252. doi: 10.1007/s11655-022-3681-x. Epub 2022 Aug 31. Chin J Integr Med. 2023. PMID: 36044117
References
-
- Anjum, R., and J. Blenis. 2008. The RSK family of kinases: emerging roles in cellular signalling. Nat. Rev. Mol. Cell Biol. 9:747-758. - PubMed
-
- Balmanno, K., S. D. Chell, A. S. Gillings, S. Hayat, and S. J. Cook. 2009. Intrinsic resistance to the MEK1/2 inhibitor AZD6244 (ARRY-142886) is associated with weak ERK1/2 signalling and/or strong PI3K signalling in colorectal cancer cell lines. Int. J. Cancer. 125:2332-2341. - PubMed
-
- Bost, F., M. Aouadi, L. Caron, P. Even, N. Belmonte, M. Prot, C. Dani, P. Hofman, G. Pages, J. Pouyssegur, Y. Le Marchand-Brustel, and B. Binetruy. 2005. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes 54:402-411. - PubMed
-
- Boulton, T. G., S. H. Nye, D. J. Robbins, N. Y. Ip, E. Radziejewska, S. D. Morgenbesser, R. A. DePinho, N. Panayotatos, M. H. Cobb, and G. D. Yancopoulos. 1991. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65:663-675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
