IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice
- PMID: 20364087
- PMCID: PMC2860944
- DOI: 10.1172/JCI40891
IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice
Abstract
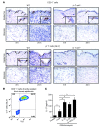
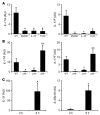
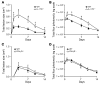
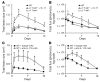
Staphylococcus aureus is the most common cause of skin and soft tissue infections, and rapidly emerging antibiotic-resistant strains are creating a serious public health concern. If immune-based therapies are to be an alternative to antibiotics, greater understanding is needed of the protective immune response against S. aureus infection in the skin. Although neutrophil recruitment is required for immunity against S. aureus, a role for T cells has been suggested. Here, we used a mouse model of S. aureus cutaneous infection to investigate the contribution of T cells to host defense. We found that mice deficient in gammadelta but not alphabeta T cells had substantially larger skin lesions with higher bacterial counts and impaired neutrophil recruitment compared with WT mice. This neutrophil recruitment was dependent upon epidermal Vgamma5+ gammadelta T cell production of IL-17, but not IL-21 and IL-22. Furthermore, IL-17 induction required IL-1, TLR2, and IL-23 and was critical for host defense, since IL-17R-deficient mice had a phenotype similar to that of gammadelta T cell-deficient mice. Importantly, gammadelta T cell-deficient mice inoculated with S. aureus and treated with a single dose of recombinant IL-17 had lesion sizes and bacterial counts resembling those of WT mice, demonstrating that IL-17 could restore the impaired immunity in these mice. Our study defines what we believe to be a novel role for IL-17-producing epidermal gammadelta T cells in innate immunity against S. aureus cutaneous infection.
Figures
Similar articles
-
Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection.J Clin Invest. 2018 Mar 1;128(3):1026-1042. doi: 10.1172/JCI96481. Epub 2018 Feb 5. J Clin Invest. 2018. PMID: 29400698 Free PMC article.
-
Chronic ethanol feeding increases the severity of Staphylococcus aureus skin infections by altering local host defenses.J Leukoc Biol. 2015 Apr;97(4):769-78. doi: 10.1189/jlb.4A0214-092R. Epub 2015 Jan 20. J Leukoc Biol. 2015. PMID: 25605871 Free PMC article.
-
Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia.BMC Immunol. 2012 Jul 9;13:38. doi: 10.1186/1471-2172-13-38. BMC Immunol. 2012. PMID: 22776294 Free PMC article.
-
Innate and adaptive immune responses against Staphylococcus aureus skin infections.Semin Immunopathol. 2012 Mar;34(2):261-80. doi: 10.1007/s00281-011-0292-6. Epub 2011 Nov 6. Semin Immunopathol. 2012. PMID: 22057887 Free PMC article. Review.
-
Immunity against Staphylococcus aureus cutaneous infections.Nat Rev Immunol. 2011 Jul 1;11(8):505-18. doi: 10.1038/nri3010. Nat Rev Immunol. 2011. PMID: 21720387 Free PMC article. Review.
Cited by
-
Host response signature to Staphylococcus aureus alpha-hemolysin implicates pulmonary Th17 response.Infect Immun. 2012 Sep;80(9):3161-9. doi: 10.1128/IAI.00191-12. Epub 2012 Jun 25. Infect Immun. 2012. PMID: 22733574 Free PMC article.
-
Conserved moonlighting protein pyruvate dehydrogenase induces robust protection against Staphylococcus aureus infection.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2321939121. doi: 10.1073/pnas.2321939121. Epub 2024 Aug 26. Proc Natl Acad Sci U S A. 2024. PMID: 39186649
-
STAT3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function.J Exp Med. 2015 Jun 1;212(6):855-64. doi: 10.1084/jem.20141992. Epub 2015 May 4. J Exp Med. 2015. PMID: 25941256 Free PMC article.
-
Staphylococcus aureus recognition by hematopoietic stem and progenitor cells via TLR2/MyD88/PGE2 stimulates granulopoiesis in wounds.Blood. 2013 Sep 5;122(10):1770-8. doi: 10.1182/blood-2012-11-466268. Epub 2013 Jul 18. Blood. 2013. PMID: 23869087 Free PMC article.
-
Biofilm-infected intracerebroventricular shunts elicit inflammation within the central nervous system.Infect Immun. 2012 Sep;80(9):3206-14. doi: 10.1128/IAI.00645-12. Epub 2012 Jul 2. Infect Immun. 2012. PMID: 22753376 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

