Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis
- PMID: 20360558
- PMCID: PMC2867693
- DOI: 10.1073/pnas.1001061107
Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis
Abstract
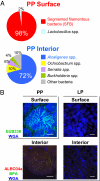
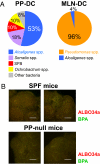
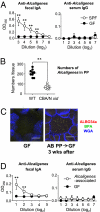
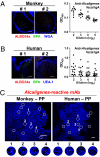
The indigenous bacteria create natural cohabitation niches together with mucosal Abs in the gastrointestinal (GI) tract. Here we report that opportunistic bacteria, largely Alcaligenes species, specifically inhabit host Peyer's patches (PPs) and isolated lymphoid follicles, with the associated preferential induction of antigen-specific mucosal IgA Abs in the GI tract. Alcaligenes were identified as the dominant bacteria on the interior of PPs from naïve, specific-pathogen-free but not from germ-free mice. Oral transfer of intratissue uncultured Alcaligenes into germ-free mice resulted in the presence of Alcaligenes inside the PPs of recipients. This result was further supported by the induction of antigen-specific Ab-producing cells in the mucosal (e.g., PPs) but not systemic compartment (e.g., spleen). The preferential presence of Alcaligenes inside PPs and the associated induction of intestinal secretory IgA Abs were also observed in both monkeys and humans. Localized mucosal Ab-mediated symbiotic immune responses were supported by Alcaligenes-stimulated CD11c(+) dendritic cells (DCs) producing the Ab-enhancing cytokines TGF-beta, B-cell-activating factor belonging to the TNF family, and IL-6 in PPs. These CD11c(+) DCs did not migrate beyond the draining mesenteric lymph nodes. In the absence of antigen-specific mucosal Abs, the presence of Alcaligenes in PPs was greatly diminished. Thus, indigenous opportunistic bacteria uniquely inhabit PPs, leading to PP-DCs-initiated, local antigen-specific Ab production; this may involve the creation of an optimal symbiotic environment on the interior of the PPs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment.J Immunol. 2007 Dec 1;179(11):7751-7. doi: 10.4049/jimmunol.179.11.7751. J Immunol. 2007. PMID: 18025221
-
Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract.J Immunol. 2000 May 15;164(10):5184-91. doi: 10.4049/jimmunol.164.10.5184. J Immunol. 2000. PMID: 10799877
-
Distinct roles for Peyer's patch B cells for induction of antigen-specific IgA antibody responses in mice administered oral recombinant Salmonella.Int Immunol. 2019 Jul 30;31(8):531-541. doi: 10.1093/intimm/dxz029. Int Immunol. 2019. PMID: 30868152
-
Peyer's patches: organizing B-cell responses at the intestinal frontier.Immunol Rev. 2016 May;271(1):230-45. doi: 10.1111/imr.12400. Immunol Rev. 2016. PMID: 27088918 Free PMC article. Review.
-
Roundtrip ticket for secretory IgA: role in mucosal homeostasis?J Immunol. 2007 Jan 1;178(1):27-32. doi: 10.4049/jimmunol.178.1.27. J Immunol. 2007. PMID: 17182536 Review.
Cited by
-
Formation of the junctions between lymph follicles in the Peyer's patches even before postweaning activation.Sci Rep. 2024 Jul 9;14(1):15783. doi: 10.1038/s41598-024-65984-4. Sci Rep. 2024. PMID: 38982122 Free PMC article.
-
A brain microbiome in salmonids at homeostasis.Sci Adv. 2024 Sep 20;10(38):eado0277. doi: 10.1126/sciadv.ado0277. Epub 2024 Sep 18. Sci Adv. 2024. PMID: 39292785 Free PMC article.
-
Gut immune maturation depends on colonization with a host-specific microbiota.Cell. 2012 Jun 22;149(7):1578-93. doi: 10.1016/j.cell.2012.04.037. Cell. 2012. PMID: 22726443 Free PMC article.
-
A subpopulation of high IL-21-producing CD4(+) T cells in Peyer's Patches is induced by the microbiota and regulates germinal centers.Sci Rep. 2016 Aug 8;6:30784. doi: 10.1038/srep30784. Sci Rep. 2016. PMID: 27499025 Free PMC article.
-
Probiotic therapy as a novel approach for allergic disease.Front Pharmacol. 2012 Sep 21;3:171. doi: 10.3389/fphar.2012.00171. eCollection 2012. Front Pharmacol. 2012. PMID: 23049509 Free PMC article.
References
-
- Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. - PubMed
-
- Cebra JJ, Jiang HQ, Boiko NV, Tlaskalva-Hogenova H. In: Mucosal Immunology. Mestecky J, et al., editors. San Diego: Academic Press; 2005. pp. 335–368.
-
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. - PubMed
-
- Kiyono H, Kunisawa J, McGhee JR, Mestecky J. In: Fundamental Immunology. Paul WE, editor. Vol 6. Philadelphia: Lippincott-Raven; 2008. pp. 983–1030.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

