Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity
- PMID: 20357360
- PMCID: PMC2889764
- DOI: 10.2337/db09-0287
Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity
Abstract
Objective: Insulin resistance and other features of the metabolic syndrome have been causally linked to adipose tissue macrophages (ATMs) in mice with diet-induced obesity. We aimed to characterize macrophage phenotype and function in human subcutaneous and omental adipose tissue in relation to insulin resistance in obesity.
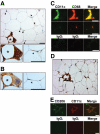
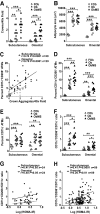
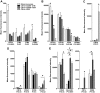
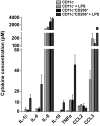
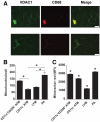
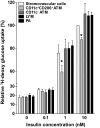
Research design and methods: Adipose tissue was obtained from lean and obese women undergoing bariatric surgery. Metabolic markers were measured in fasting serum and ATMs characterized by immunohistology, flow cytometry, and tissue culture studies. RESULTS ATMs comprised CD11c(+)CD206(+) cells in "crown" aggregates and solitary CD11c(-)CD206(+) cells at adipocyte junctions. In obese women, CD11c(+) ATM density was greater in subcutaneous than omental adipose tissue and correlated with markers of insulin resistance. CD11c(+) ATMs were distinguished by high expression of integrins and antigen presentation molecules; interleukin (IL)-1beta, -6, -8, and -10; tumor necrosis factor-alpha; and CC chemokine ligand-3, indicative of an activated, proinflammatory state. In addition, CD11c(+) ATMs were enriched for mitochondria and for RNA transcripts encoding mitochondrial, proteasomal, and lysosomal proteins, fatty acid metabolism enzymes, and T-cell chemoattractants, whereas CD11c(-) ATMs were enriched for transcripts involved in tissue maintenance and repair. Tissue culture medium conditioned by CD11c(+) ATMs, but not CD11c(-) ATMs or other stromovascular cells, impaired insulin-stimulated glucose uptake by human adipocytes.
Conclusions: These findings identify proinflammatory CD11c(+) ATMs as markers of insulin resistance in human obesity. In addition, the machinery of CD11c(+) ATMs indicates they metabolize lipid and may initiate adaptive immune responses.
Figures

Similar articles
-
Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice.J Biol Chem. 2010 May 14;285(20):15333-15345. doi: 10.1074/jbc.M110.100263. Epub 2010 Mar 22. J Biol Chem. 2010. PMID: 20308074 Free PMC article.
-
Lack of oncostatin M receptor β leads to adipose tissue inflammation and insulin resistance by switching macrophage phenotype.J Biol Chem. 2013 Jul 26;288(30):21861-75. doi: 10.1074/jbc.M113.461905. Epub 2013 Jun 11. J Biol Chem. 2013. PMID: 23760275 Free PMC article.
-
Human CD206+ macrophages associate with diabetes and adipose tissue lymphoid clusters.JCI Insight. 2022 Feb 8;7(3):e146563. doi: 10.1172/jci.insight.146563. JCI Insight. 2022. PMID: 34990410 Free PMC article.
-
Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance.Biochim Biophys Acta. 2014 Mar;1842(3):446-62. doi: 10.1016/j.bbadis.2013.05.017. Epub 2013 May 22. Biochim Biophys Acta. 2014. PMID: 23707515 Free PMC article. Review.
-
Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states.Curr Opin Clin Nutr Metab Care. 2011 Jul;14(4):341-6. doi: 10.1097/MCO.0b013e328347970b. Curr Opin Clin Nutr Metab Care. 2011. PMID: 21587064 Free PMC article. Review.
Cited by
-
Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease.J Innate Immun. 2022;14(1):4-30. doi: 10.1159/000515117. Epub 2021 Apr 13. J Innate Immun. 2022. PMID: 33849008 Free PMC article. Review.
-
Concise Review: Macrophages: Versatile Gatekeepers During Pancreatic β-Cell Development, Injury, and Regeneration.Stem Cells Transl Med. 2015 Jun;4(6):555-63. doi: 10.5966/sctm.2014-0272. Epub 2015 Apr 6. Stem Cells Transl Med. 2015. PMID: 25848123 Free PMC article. Review.
-
A review on the biology and properties of adipose tissue macrophages involved in adipose tissue physiological and pathophysiological processes.Lipids Health Dis. 2020 Jul 9;19(1):164. doi: 10.1186/s12944-020-01342-3. Lipids Health Dis. 2020. PMID: 32646451 Free PMC article. Review.
-
Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2.Nat Commun. 2022 Sep 5;13(1):5208. doi: 10.1038/s41467-022-32871-3. Nat Commun. 2022. PMID: 36064857 Free PMC article.
-
Adipose Tissue Dendritic Cells Are Independent Contributors to Obesity-Induced Inflammation and Insulin Resistance.J Immunol. 2016 Nov 1;197(9):3650-3661. doi: 10.4049/jimmunol.1600820. Epub 2016 Sep 28. J Immunol. 2016. PMID: 27683748 Free PMC article.
References
-
- O'Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, Strauss B, Marks S, Schachter L, Chapman L, Anderson M: Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 2006;144:625–633 - PubMed
-
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS: Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007;56:2910–2918 - PubMed
-
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M: IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198 - PubMed
-
- Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M: Macrophage PPARgamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 2007;117:1658–1669 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

