Synaptic mechanism for functional synergism between delta- and mu-opioid receptors
- PMID: 20357124
- PMCID: PMC2858689
- DOI: 10.1523/JNEUROSCI.5968-09.2010
Synaptic mechanism for functional synergism between delta- and mu-opioid receptors
Abstract
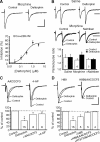
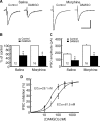
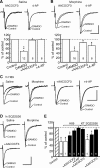
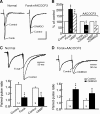
By sustained activation of mu-opioid receptors (MORs), chronic opioids cause analgesic tolerance, physical dependence, and opioid addiction, common clinical problems for which an effective treatment is still lacking. Chronic opioids recruit delta-opioid receptors (DORs) to plasma membrane through exocytotic trafficking, but the role of this new DOR and its interaction with existing MOR in brain functions and in these clinical problems remain largely unknown. In this study, we investigated the mechanisms underlying synaptic and behavioral actions of chronic morphine-induced DORs and their interaction with MORs in nucleus raphe magnus (NRM) neurons important for opioid analgesia. We found that the emerged DOR inhibited GABAergic IPSCs through both the phospholipase A(2) (PLA(2)) and cAMP/protein kinase A (PKA) signaling pathways. MOR inhibition of IPSCs, normally mediated predominantly by the PLA(2) pathway, was additionally mediated by the cAMP/PKA pathway, with MOR potency significantly increased after chronic morphine treatment. Isobologram analysis revealed a synergistic DOR-MOR interaction in their IPSC inhibition, which was dependent on upregulated activities of both the PLA(2) and cAMP/PKA pathways. Furthermore, DOR and MOR agonists microinjected into the NRM in vivo also produced a PLA(2)-dependent synergism in their antinociceptive effects. These findings suggest that the cAMP/PKA pathway, upregulated by chronic opioids, becomes more important in the mechanisms of both MOR and DOR inhibition of GABA synaptic transmission after chronic opioid exposure, and DORs and MORs are synergic both synaptically and behaviorally in producing analgesic effects in a PLA(2)-dependent fashion, supporting the potential therapeutic use of DOR agonists in pain management under chronic opioid conditions.
Figures
Similar articles
-
Signaling cascades for δ-opioid receptor-mediated inhibition of GABA synaptic transmission and behavioral antinociception.Mol Pharmacol. 2012 Mar;81(3):375-83. doi: 10.1124/mol.111.076307. Epub 2011 Dec 5. Mol Pharmacol. 2012. PMID: 22144670 Free PMC article.
-
Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses.J Pharmacol Exp Ther. 2009 Apr;329(1):290-6. doi: 10.1124/jpet.108.148908. Epub 2009 Jan 23. J Pharmacol Exp Ther. 2009. PMID: 19168708 Free PMC article.
-
Histone deacetylase inhibitor-induced emergence of synaptic δ-opioid receptors and behavioral antinociception in persistent neuropathic pain.Neuroscience. 2016 Dec 17;339:54-63. doi: 10.1016/j.neuroscience.2016.09.015. Epub 2016 Sep 17. Neuroscience. 2016. PMID: 27646288
-
Interaction and regulatory functions of μ- and δ-opioid receptors in nociceptive afferent neurons.Neurosci Bull. 2012 Apr;28(2):121-30. doi: 10.1007/s12264-012-1206-x. Neurosci Bull. 2012. PMID: 22466123 Free PMC article. Review.
-
Do pharmacological approaches that prevent opioid tolerance target different elements in the same regulatory machinery?Curr Drug Abuse Rev. 2008 Jun;1(2):222-38. doi: 10.2174/1874473710801020222. Curr Drug Abuse Rev. 2008. PMID: 19630721 Review.
Cited by
-
Molecular Pharmacology of δ-Opioid Receptors.Pharmacol Rev. 2016 Jul;68(3):631-700. doi: 10.1124/pr.114.008979. Pharmacol Rev. 2016. PMID: 27343248 Free PMC article. Review.
-
Heteromerization Modulates mu Opioid Receptor Functional Properties in vivo.Front Pharmacol. 2018 Nov 13;9:1240. doi: 10.3389/fphar.2018.01240. eCollection 2018. Front Pharmacol. 2018. PMID: 30483121 Free PMC article. Review.
-
Opium alkaloids, biosynthesis, pharmacology and association with cancer occurrence.Open Biol. 2023 May;13(5):220355. doi: 10.1098/rsob.220355. Epub 2023 May 3. Open Biol. 2023. PMID: 37132222 Free PMC article. Review.
-
Generation and Characterization of Antibodies against Opioid Receptors from Zebrafish.Int J Mol Sci. 2018 Jan 2;19(1):14. doi: 10.3390/ijms19010014. Int J Mol Sci. 2018. PMID: 29301275 Free PMC article.
-
A novel opioid receptor-mediated enhancement of GABAA receptor function induced by stress in ventral tegmental area neurons.J Physiol. 2011 Sep 1;589(17):4229-42. doi: 10.1113/jphysiol.2011.209023. Epub 2011 Jun 20. J Physiol. 2011. PMID: 21690191 Free PMC article.
References
-
- Adams JU, Tallarida RJ, Geller EB, Adler MW. Isobolographic superadditivity between delta and mu opioid agonists in the rat depends on the ratio of compounds, the mu agonist and the analgesic assay used. J Pharmacol Exp Ther. 1993;266:1261–1267. - PubMed
-
- Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. - PubMed
-
- Bhargava HN. Diversity of agents that modify opioid tolerance, physical dependence, abstinence syndrome, and self-administrative behavior. Pharmacol Rev. 1994;46:293–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
