An integrated model for enzyme catalysis emerges from studies of hydrogen tunneling
- PMID: 20354595
- PMCID: PMC2846846
- DOI: 10.1016/j.cplett.2009.01.038
An integrated model for enzyme catalysis emerges from studies of hydrogen tunneling
Abstract
The origins of the enormous rate accelerations brought about by enzymes are discussed. The focus is on enzymatic C-H activation, which has been shown to take place via tunneling. Four enzyme systems illustrate the impact of site-specific mutagenesis, changes in temperature or changes in protein solvation on the tunneling properties. A model emerges in which conformational sampling is required to access a subset of protein conformers where the H-donor and acceptor undergo a close approach. The evidence for an inverse relationship between protein flexibility and active site compression is likely to extend to all classes of enzyme catalysts.
Figures

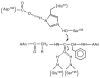

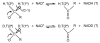
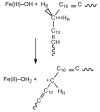
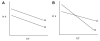

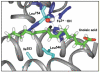
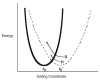
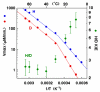
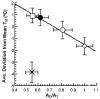
 , D and
, D and  , H) and of the H/D KIE (
, H) and of the H/D KIE ( ) for the ht-ADH.
) for the ht-ADH.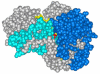


Similar articles
-
Understanding Biological Hydrogen Transfer Through the Lens of Temperature Dependent Kinetic Isotope Effects.Acc Chem Res. 2018 Sep 18;51(9):1966-1974. doi: 10.1021/acs.accounts.8b00226. Epub 2018 Aug 28. Acc Chem Res. 2018. PMID: 30152685 Free PMC article. Review.
-
Extremely elevated room-temperature kinetic isotope effects quantify the critical role of barrier width in enzymatic C-H activation.J Am Chem Soc. 2014 Jun 11;136(23):8157-60. doi: 10.1021/ja502726s. Epub 2014 Jun 2. J Am Chem Soc. 2014. PMID: 24884374 Free PMC article.
-
The role of tunneling in enzyme catalysis of C-H activation.Biochim Biophys Acta. 2006 Aug;1757(8):981-7. doi: 10.1016/j.bbabio.2005.12.004. Epub 2006 Feb 8. Biochim Biophys Acta. 2006. PMID: 16546116 Review.
-
13C ENDOR Spectroscopy of Lipoxygenase-Substrate Complexes Reveals the Structural Basis for C-H Activation by Tunneling.J Am Chem Soc. 2017 Feb 8;139(5):1984-1997. doi: 10.1021/jacs.6b11856. Epub 2017 Jan 25. J Am Chem Soc. 2017. PMID: 28121140 Free PMC article.
-
Dynamically achieved active site precision in enzyme catalysis.Acc Chem Res. 2015 Feb 17;48(2):449-56. doi: 10.1021/ar5003347. Epub 2014 Dec 24. Acc Chem Res. 2015. PMID: 25539048 Free PMC article. Review.
Cited by
-
Determinants of Photolyase's DNA Repair Mechanism in Mesophiles and Extremophiles.J Am Chem Soc. 2018 Feb 28;140(8):2853-2861. doi: 10.1021/jacs.7b11926. Epub 2018 Feb 13. J Am Chem Soc. 2018. PMID: 29401372 Free PMC article.
-
Submillisecond mixing in a continuous-flow, microfluidic mixer utilizing mid-infrared hyperspectral imaging detection.Lab Chip. 2014 Feb 7;14(3):584-91. doi: 10.1039/c3lc51171e. Lab Chip. 2014. PMID: 24302515 Free PMC article.
-
Enzymatic methyl transfer: role of an active site residue in generating active site compaction that correlates with catalytic efficiency.J Am Chem Soc. 2011 Nov 2;133(43):17134-7. doi: 10.1021/ja207467d. Epub 2011 Oct 10. J Am Chem Soc. 2011. PMID: 21958159 Free PMC article.
-
Computational identification of slow conformational fluctuations in proteins.J Phys Chem B. 2009 Dec 31;113(52):16669-80. doi: 10.1021/jp9077213. J Phys Chem B. 2009. PMID: 19908896 Free PMC article.
-
Enzymatic transition states and dynamic motion in barrier crossing.Nat Chem Biol. 2009 Aug;5(8):551-8. doi: 10.1038/nchembio.202. Nat Chem Biol. 2009. PMID: 19620996 Free PMC article.
References
-
- Wolfenden R, Snider MJ. Acc. Chem. Res. 2001;34:938. - PubMed
-
- Dwyer MA, Looger LL, Hellinga HW. Science. 2008;319:569. - PubMed
-
- Jiang L, et al. Science. 2005;309:1868. - PubMed
-
- Pauling L. Am. Sci. 1948;36:51. - PubMed
-
- Wolfenden R. In: Transition States of Biochemical Processes. Gandour RD, Schowen RL, editors. Plenum Press; New York and London: 1978. p. 558.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
