Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2
- PMID: 20352235
- PMCID: PMC2916114
- DOI: 10.1007/s00395-010-0098-z
Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2
Abstract
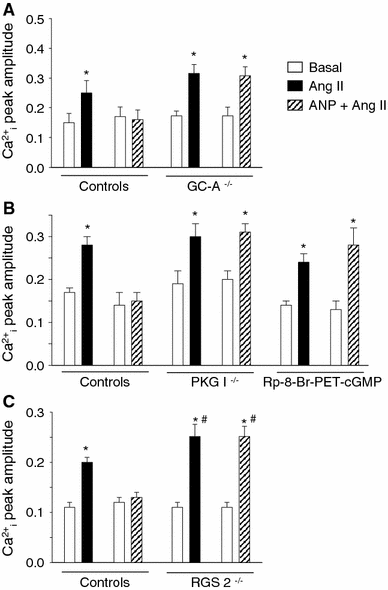
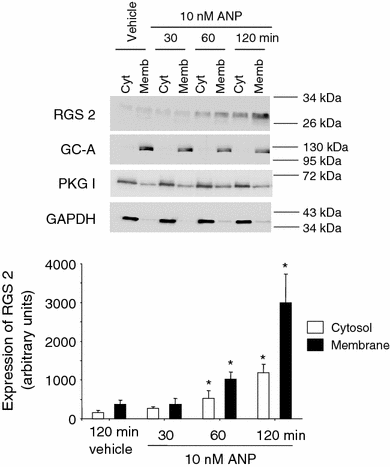
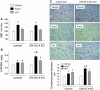
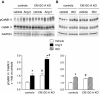
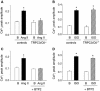
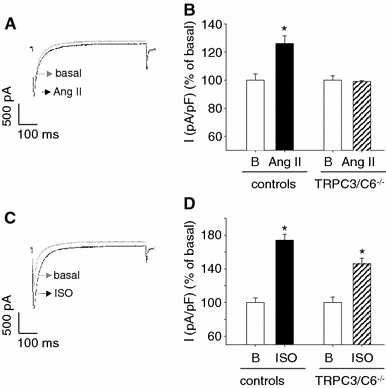
Cardiac atrial natriuretic peptide (ANP) locally counteracts cardiac hypertrophy via the guanylyl cyclase-A (GC-A) receptor and cGMP production, but the downstream signalling pathways are unknown. Here, we examined the influence of ANP on beta-adrenergic versus Angiotensin II (Ang II)-dependent (G(s) vs. G(alphaq) mediated) modulation of Ca(2+) (i)-handling in cardiomyocytes and of hypertrophy in intact hearts. L-type Ca(2+) currents and Ca(2+) (i) transients in adult isolated murine ventricular myocytes were studied by voltage-clamp recordings and fluorescence microscopy. ANP suppressed Ang II-stimulated Ca(2+) currents and transients, but had no effect on isoproterenol stimulation. Ang II suppression by ANP was abolished in cardiomyocytes of mice deficient in GC-A, in cyclic GMP-dependent protein kinase I (PKG I) or in the regulator of G protein signalling (RGS) 2, a target of PKG I. Cardiac hypertrophy in response to exogenous Ang II was significantly exacerbated in mice with conditional, cardiomyocyte-restricted GC-A deletion (CM GC-A KO). This was concomitant to increased activation of the Ca(2+)/calmodulin-dependent prohypertrophic signal transducer CaMKII. In contrast, beta-adrenoreceptor-induced hypertrophy was not enhanced in CM GC-A KO mice. Lastly, while the stimulatory effects of Ang II on Ca(2+)-handling were absent in myocytes of mice deficient in TRPC3/TRPC6, the effects of isoproterenol were unchanged. Our data demonstrate a direct myocardial role for ANP/GC-A/cGMP to antagonize the Ca(2+) (i)-dependent hypertrophic growth response to Ang II, but not to beta-adrenergic stimulation. The selectivity of this interaction is determined by PKG I and RGS2-dependent modulation of Ang II/AT(1) signalling. Furthermore, they strengthen published observations in neonatal cardiomyocytes showing that TRPC3/TRPC6 channels are essential for Ang II, but not for beta-adrenergic Ca(2+) (i)-stimulation in adult myocytes.
Figures
Similar articles
-
Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart.Circ Res. 2010 Jun 25;106(12):1849-60. doi: 10.1161/CIRCRESAHA.109.208314. Epub 2010 May 6. Circ Res. 2010. PMID: 20448219
-
CaMKII-mediated increased lusitropic responses to beta-adrenoreceptor stimulation in ANP-receptor deficient mice.Cardiovasc Res. 2007 Mar 1;73(4):678-88. doi: 10.1016/j.cardiores.2006.10.003. Epub 2006 Oct 7. Cardiovasc Res. 2007. PMID: 17107670
-
Atrial natriuretic peptide-mediated inhibition of microcirculatory endothelial Ca2+ and permeability response to histamine involves cGMP-dependent protein kinase I and TRPC6 channels.Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2121-9. doi: 10.1161/ATVBAHA.113.001974. Epub 2013 Jun 27. Arterioscler Thromb Vasc Biol. 2013. PMID: 23814119
-
Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels.Comp Biochem Physiol A Mol Integr Physiol. 2005 Oct;142(2):136-43. doi: 10.1016/j.cbpb.2005.04.012. Epub 2005 May 31. Comp Biochem Physiol A Mol Integr Physiol. 2005. PMID: 15927494 Review.
-
Angiotensin II-Induced Signal Transduction Mechanisms for Cardiac Hypertrophy.Cells. 2022 Oct 22;11(21):3336. doi: 10.3390/cells11213336. Cells. 2022. PMID: 36359731 Free PMC article. Review.
Cited by
-
Natriuretic Peptide Signaling in Uterine Biology and Preeclampsia.Int J Mol Sci. 2023 Aug 1;24(15):12309. doi: 10.3390/ijms241512309. Int J Mol Sci. 2023. PMID: 37569683 Free PMC article. Review.
-
Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides.J Mol Med (Berl). 2012 Jan;90(1):5-13. doi: 10.1007/s00109-011-0801-z. Epub 2011 Aug 9. J Mol Med (Berl). 2012. PMID: 21826523 Review.
-
The Impact of Natriuretic Peptides on Heart Development, Homeostasis, and Disease.Cells. 2024 May 28;13(11):931. doi: 10.3390/cells13110931. Cells. 2024. PMID: 38891063 Free PMC article. Review.
-
Decoding signaling mechanisms: unraveling the targets of guanylate cyclase agonists in cardiovascular and digestive diseases.Front Pharmacol. 2023 Dec 20;14:1272073. doi: 10.3389/fphar.2023.1272073. eCollection 2023. Front Pharmacol. 2023. PMID: 38186653 Free PMC article. Review.
-
Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart.Basic Res Cardiol. 2011 Nov;106(6):1023-39. doi: 10.1007/s00395-011-0228-2. Epub 2011 Oct 20. Basic Res Cardiol. 2011. PMID: 22012077 Free PMC article.
References
-
- Dietrich A, Mederos Y, Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

