IL-17 enhancement of the IL-6 signaling cascade in astrocytes
- PMID: 20351184
- PMCID: PMC3769161
- DOI: 10.4049/jimmunol.1000142
IL-17 enhancement of the IL-6 signaling cascade in astrocytes
Abstract
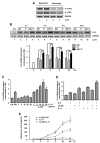
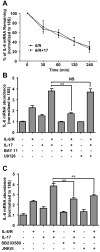
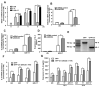
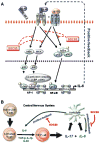
Astrocytes have important physiological roles in CNS homeostasis and serve as a bridge between the CNS and immune system. IL-17 and IL-6 are important in many CNS disorders characterized by neuroinflammation. We examined the role of IL-17 on the IL-6 signaling cascade in primary astrocytes. IL-17 functioned in a synergistic manner with IL-6 to induce IL-6 expression in astrocytes. The synergistic effect involved numerous signaling pathways including NF-kappaB, JNK MAPK, and p38 MAPK. The NF-kappaB pathway inhibitor BAY-11, JNK inhibitor JNKi II, and p38 inhibitor SB203580 suppressed the synergistic effect of IL-6 and IL-17 on IL-6 expression. IL-17 synergized with IL-6 to enhance the recruitment of activated NF-kappaB p65, c-Fos, c-Jun, and the histone acetyltransferases CREB-binding protein and p300 to the IL-6 promoter in vivo to induce IL-6 transcription. This was accompanied by enhanced acetylation of histones H3 and H4 on the IL-6 promoter. Moreover, we elucidated an important role for suppressor of cytokine signaling (SOCS) 3 in IL-17 enhancement of IL-6 signaling in astrocytes. SOCS3 small interfering RNA knockdown and SOCS3 deletion in astrocytes augmented the synergistic effect of IL-6 and IL-17 due to an enhancement of activation of the NF-kappaB and MAPK pathways. These results indicate that astrocytes can serve as a target of Th17 cells and IL-17 in the CNS, and SOCS3 participates in IL-17 functions in the CNS as a negative feedback regulator.
Figures



Similar articles
-
TGF-beta promotes Th17 cell development through inhibition of SOCS3.J Immunol. 2009 Jul 1;183(1):97-105. doi: 10.4049/jimmunol.0801986. Epub 2009 Jun 17. J Immunol. 2009. PMID: 19535626 Free PMC article.
-
Regulation of CCL20 expression in astrocytes by IL-6 and IL-17.Glia. 2012 May;60(5):771-81. doi: 10.1002/glia.22307. Epub 2012 Feb 8. Glia. 2012. PMID: 22319003
-
Molecular basis of oncostatin M-induced SOCS-3 expression in astrocytes.Glia. 2008 Aug 15;56(11):1250-62. doi: 10.1002/glia.20694. Glia. 2008. PMID: 18571793 Free PMC article.
-
Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia.J Immunol. 2007 Nov 1;179(9):5966-76. doi: 10.4049/jimmunol.179.9.5966. J Immunol. 2007. PMID: 17947670
-
Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation.J Immunol. 2002 Jun 15;168(12):6404-11. doi: 10.4049/jimmunol.168.12.6404. J Immunol. 2002. PMID: 12055259
Cited by
-
Immune players in the CNS: the astrocyte.J Neuroimmune Pharmacol. 2013 Sep;8(4):824-39. doi: 10.1007/s11481-013-9480-6. Epub 2013 Jul 4. J Neuroimmune Pharmacol. 2013. PMID: 23821340 Review.
-
IFN-γ-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain.J Immunol. 2012 Jul 15;189(2):968-79. doi: 10.4049/jimmunol.1200688. Epub 2012 Jun 20. J Immunol. 2012. PMID: 22723523 Free PMC article.
-
TLR2/4 deficiency prevents oxygen-induced vascular degeneration and promotes revascularization by downregulating IL-17 in the retina.Sci Rep. 2016 Jun 14;6:27739. doi: 10.1038/srep27739. Sci Rep. 2016. PMID: 27297042 Free PMC article.
-
Neurocognitive Profile of the Post-COVID Condition in Adults in Catalonia-A Mixed Method Prospective Cohort and Nested Case-Control Study: Study Protocol.Vaccines (Basel). 2022 May 26;10(6):849. doi: 10.3390/vaccines10060849. Vaccines (Basel). 2022. PMID: 35746457 Free PMC article.
-
Interleukin-6 in Traumatic Brain Injury: A Janus-Faced Player in Damage and Repair.J Neurotrauma. 2023 Nov;40(21-22):2249-2269. doi: 10.1089/neu.2023.0135. Epub 2023 Aug 10. J Neurotrauma. 2023. PMID: 37166354 Free PMC article. Review.
References
-
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. - PubMed
-
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. - PubMed
-
- Williams A, Piaton G, Lubetzki C. Astrocytes--friends or foes in multiple sclerosis? Glia. 2007;55:1300–1312. - PubMed
-
- Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature. 2009;457:675–677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

