The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes
- PMID: 20345488
- PMCID: PMC2923681
- DOI: 10.1111/j.1462-5822.2010.01468.x
The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes
Abstract
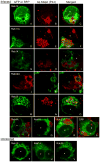
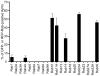
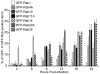
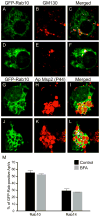
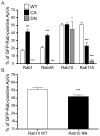
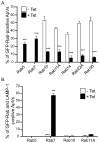
Anaplasma phagocytophilum is an obligate intracellular bacterium that infects neutrophils to reside within a host cell-derived vacuole. The A. phagocytophilum-occupied vacuole (ApV) fails to mature along the endocytic pathway and is non-fusogenic with lysosomes. Rab GTPases regulate membrane traffic. To better understand how the bacterium modulates the ApV's selective fusogencity, we examined the intracellular localization of 20 green fluorescent protein (GFP) or red fluorescent protein (RFP)-tagged Rab GTPases in A. phagocytophilum-infected HL-60 cells. GFP-Rab4A, GFP-Rab10, GFP-Rab11A, GFP-Rab14, RFP-Rab22A and GFP-Rab35, which regulate endocytic recycling, and GFP-Rab1, which mediates endoplasmic reticulum to Golgi apparatus trafficking, localize to the ApV. Fluorescently tagged Rabs are recruited to the ApV upon its formation and remain associated throughout infection. Endogenous Rab14 localizes to the ApV. Tetracycline treatment concomitantly promotes loss of recycling endosome-associated GFP-Rabs and acquisition of GFP-Rab5, GFP-Rab7, and the lysosomal marker, LAMP-1. Wild-type and GTPase- deficient versions, but not GDP-restricted versions of GFP-Rab1, GFP-Rab4A and GFP-Rab11A, localize to the ApV. Strikingly, GFP-Rab10 recruitment to the ApV is guanine nucleotide-independent. These data establish that A. phagocytophilum selectively recruits Rab GTPases that are primarily associated with recycling endosomes to facilitate its intracellular survival and implicate bacterial proteins in regulating Rab10 membrane cycling on the ApV.
Figures

Similar articles
-
Breaking in and grabbing a meal: Anaplasma phagocytophilum cellular invasion, nutrient acquisition, and promising tools for their study.Microbes Infect. 2013 Dec;15(14-15):1017-25. doi: 10.1016/j.micinf.2013.10.010. Epub 2013 Oct 18. Microbes Infect. 2013. PMID: 24141091 Free PMC article. Review.
-
The Pathogen-Occupied Vacuoles of Anaplasma phagocytophilum and Anaplasma marginale Interact with the Endoplasmic Reticulum.Front Cell Infect Microbiol. 2016 Mar 1;6:22. doi: 10.3389/fcimb.2016.00022. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26973816 Free PMC article.
-
Anaplasma phagocytophilum Rab10-dependent parasitism of the trans-Golgi network is critical for completion of the infection cycle.Cell Microbiol. 2016 Feb;18(2):260-81. doi: 10.1111/cmi.12500. Epub 2015 Oct 7. Cell Microbiol. 2016. PMID: 26289115 Free PMC article.
-
Coxiella burnetii-containing vacuoles interact with host recycling endosomal proteins Rab11a and Rab35 for vacuolar expansion and bacterial growth.Front Cell Infect Microbiol. 2024 May 22;14:1394019. doi: 10.3389/fcimb.2024.1394019. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38841112 Free PMC article.
-
[Rab GTPases networks in membrane traffic in Saccharomyces cerevisiae].Yakugaku Zasshi. 2015;135(3):483-92. doi: 10.1248/yakushi.14-00246. Yakugaku Zasshi. 2015. PMID: 25759056 Review. Japanese.
Cited by
-
Immunization against Anaplasma phagocytophilum Adhesin Binding Domains Confers Protection against Infection in the Mouse Model.Infect Immun. 2020 Sep 18;88(10):e00106-20. doi: 10.1128/IAI.00106-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32661123 Free PMC article.
-
Anaplasma phagocytophilum APH0032 Is Exposed on the Cytosolic Face of the Pathogen-Occupied Vacuole and Co-opts Host Cell SUMOylation.Front Cell Infect Microbiol. 2016 Sep 22;6:108. doi: 10.3389/fcimb.2016.00108. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27713867 Free PMC article.
-
Breaking in and grabbing a meal: Anaplasma phagocytophilum cellular invasion, nutrient acquisition, and promising tools for their study.Microbes Infect. 2013 Dec;15(14-15):1017-25. doi: 10.1016/j.micinf.2013.10.010. Epub 2013 Oct 18. Microbes Infect. 2013. PMID: 24141091 Free PMC article. Review.
-
Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport.PLoS Pathog. 2014 Mar 13;10(3):e1003995. doi: 10.1371/journal.ppat.1003995. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24626429 Free PMC article.
-
Deviant Behavior: Tick-Borne Pathogens and Inflammasome Signaling.Vet Sci. 2016 Sep 28;3(4):27. doi: 10.3390/vetsci3040027. Vet Sci. 2016. PMID: 29056735 Free PMC article. Review.
References
-
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. - PubMed
-
- Borjesson DL, Kobayashi SD, Whitney AR, Voyich JM, Argue CM, Deleo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. 2005;174:6364–6372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54-AI057158/AI/NIAID NIH HHS/United States
- R01 AI072683/AI/NIAID NIH HHS/United States
- P30 NS047463/NS/NINDS NIH HHS/United States
- AI072683/AI/NIAID NIH HHS/United States
- R56 AI072683/AI/NIAID NIH HHS/United States
- R01 AI041699/AI/NIAID NIH HHS/United States
- F32-AI082927/AI/NIAID NIH HHS/United States
- 5P30NS047463/NS/NINDS NIH HHS/United States
- R01 AI072683-04/AI/NIAID NIH HHS/United States
- R37 AI072683/AI/NIAID NIH HHS/United States
- R01 AI072683-03/AI/NIAID NIH HHS/United States
- AI073831/AI/NIAID NIH HHS/United States
- R01 AI041699-15/AI/NIAID NIH HHS/United States
- R01 AI064559-05/AI/NIAID NIH HHS/United States
- R01 AI073831/AI/NIAID NIH HHS/United States
- R01 AI064559/AI/NIAID NIH HHS/United States
- R01-AI064559/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

