A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion
- PMID: 20339555
- PMCID: PMC2842440
- DOI: 10.1371/journal.pone.0009808
A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion
Abstract
Background: Glioblastoma multiforme (GBM, WHO grade IV) is the most common and most malignant of astrocytic brain tumors, and is associated with rapid invasion into neighboring tissue. In other tumor types it is well established that such invasion involves a complex interaction between tumor cells and locally produced extracellular matrix. In GBMs, surprisingly little is known about the associated matrix components, in particular the fibrillar proteins such as collagens that are known to play a key role in the invasion of other tumor types.
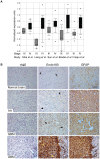
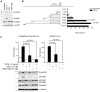
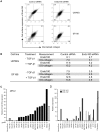
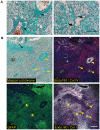
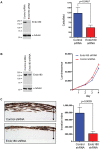
Methodology/principal findings: In this study we have used both the Masson's trichrome staining and a high resolution multiple immunofluorescence labeling method to demonstrate that intratumoral fibrillar collagens are an integral part of the extracellular matrix in a subset of GBMs. Correlated with this collagen deposition we observed high level expression of the collagen-binding receptor Endo180 (CD280) in the tumor cells. Further, interrogation of multiple expression array datasets identified Endo180 as one of the most highly upregulated transcripts in grade IV GBMs compared to grade III gliomas. Using promoter analysis studies we show that this increased expression is, in part, mediated via TGF-beta signaling. Functionally, we demonstrate that Endo180 serves as the major collagen internalization receptor in GBM cell lines and provide the first evidence that this activity is critical for the invasion of GBM cells through fibrillar collagen matrices.
Conclusions/significance: This study demonstrates, for the first time, that fibrillar collagens are extensively deposited in GBMs and that the collagen internalization receptor Endo180 is both highly expressed in these tumors and that it serves to mediate the invasion of tumor cells through collagen-containing matrices. Together these data provide important insights into the mechanism of GBM invasion and identify Endo180 as a potential target to limit matrix turnover by glioma cells and thereby restrict tumor progression.
Conflict of interest statement
Figures
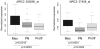
Similar articles
-
The collagen receptor uPARAP/Endo180 in tissue degradation and cancer (Review).Int J Oncol. 2015 Oct;47(4):1177-88. doi: 10.3892/ijo.2015.3120. Epub 2015 Aug 12. Int J Oncol. 2015. PMID: 26316068 Free PMC article. Review.
-
The collagen receptor Endo180 (CD280) Is expressed on basal-like breast tumor cells and promotes tumor growth in vivo.Cancer Res. 2007 Nov 1;67(21):10230-40. doi: 10.1158/0008-5472.CAN-06-3496. Cancer Res. 2007. PMID: 17974964
-
TGFβ1-Endo180-dependent collagen deposition is dysregulated at the tumour-stromal interface in bone metastasis.J Pathol. 2012 Apr;226(5):775-83. doi: 10.1002/path.3958. Epub 2011 Dec 8. J Pathol. 2012. PMID: 22072289
-
Membrane type-1 matrix metalloproteinase activity is regulated by the endocytic collagen receptor Endo180.J Cell Sci. 2009 Nov 15;122(Pt 22):4042-8. doi: 10.1242/jcs.044305. Epub 2009 Oct 27. J Cell Sci. 2009. PMID: 19861500 Free PMC article.
-
The collagen receptor uPARAP/Endo180.Front Biosci (Landmark Ed). 2009 Jan 1;14(6):2103-14. doi: 10.2741/3365. Front Biosci (Landmark Ed). 2009. PMID: 19273187 Review.
Cited by
-
Impairment of a distinct cancer-associated fibroblast population limits tumour growth and metastasis.Nat Commun. 2021 Jun 10;12(1):3516. doi: 10.1038/s41467-021-23583-1. Nat Commun. 2021. PMID: 34112782 Free PMC article.
-
The mechanical and pharmacological regulation of glioblastoma cell migration in 3D matrices.J Cell Physiol. 2019 Apr;234(4):3948-3960. doi: 10.1002/jcp.27209. Epub 2018 Aug 21. J Cell Physiol. 2019. PMID: 30132879 Free PMC article.
-
The collagen receptor uPARAP/Endo180 in tissue degradation and cancer (Review).Int J Oncol. 2015 Oct;47(4):1177-88. doi: 10.3892/ijo.2015.3120. Epub 2015 Aug 12. Int J Oncol. 2015. PMID: 26316068 Free PMC article. Review.
-
Development of a Synthetic, Injectable Hydrogel to Capture Residual Glioblastoma and Glioblastoma Stem-Like Cells with CXCL12-Mediated Chemotaxis.Adv Healthc Mater. 2023 Jun;12(14):e2300671. doi: 10.1002/adhm.202300671. Epub 2023 Apr 20. Adv Healthc Mater. 2023. PMID: 37014179 Free PMC article.
-
Modeling extracellular matrix reorganization in 3D environments.PLoS One. 2013;8(1):e52509. doi: 10.1371/journal.pone.0052509. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23341900 Free PMC article.
References
-
- D'Abaco GM, Kaye AH. Integrins: molecular determinants of glioma invasion. J Clin Neurosci. 2007;14:1041–1048. - PubMed
-
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. - PubMed
-
- Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

