The C. elegans homolog of nucleoporin Nup98 is required for the integrity and function of germline P granules
- PMID: 20335358
- PMCID: PMC2853846
- DOI: 10.1242/dev.047654
The C. elegans homolog of nucleoporin Nup98 is required for the integrity and function of germline P granules
Abstract
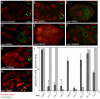
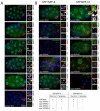
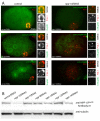
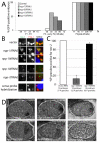
C. elegans P granules are conserved cytoplasmic ribonucleoprotein complexes that are unique to the germline and essential for fertility. During most of germline development, P granules are perinuclear and associate with clusters of nuclear pores. In an RNAi screen against nucleoporins, we have identified a specific nucleoporin essential for P granule integrity and function. The C. elegans homolog of vertebrate Nup98 (CeNup98) is enriched in P granules and associates with the translationally repressed, P granule-enriched mRNA nos-2 (nanos homolog). Loss of CeNup98 causes P granules to disperse in the cytoplasm and to release nos-2 mRNA. Embryos depleted for CeNup98 express a nos-2 3'UTR reporter prematurely. In the mouse, Nup98 immunoprecipitates with the germ granule component MVH. Our findings suggest that, in germ cells, the function of Nup98 extends beyond transport at the nuclear pore to include mRNA regulation in the cytoplasm.
Figures


Similar articles
-
ELLI-1, a novel germline protein, modulates RNAi activity and P-granule accumulation in Caenorhabditis elegans.PLoS Genet. 2017 Feb 9;13(2):e1006611. doi: 10.1371/journal.pgen.1006611. eCollection 2017 Feb. PLoS Genet. 2017. PMID: 28182654 Free PMC article.
-
The DEAD box RNA helicase VBH-1 is required for germ cell function in C. elegans.Genesis. 2007 Sep;45(9):533-46. doi: 10.1002/dvg.20323. Genesis. 2007. PMID: 17868112
-
A genomewide RNAi screen for genes that affect the stability, distribution and function of P granules in Caenorhabditis elegans.Genetics. 2009 Dec;183(4):1397-419. doi: 10.1534/genetics.109.110171. Epub 2009 Oct 5. Genetics. 2009. PMID: 19805813 Free PMC article.
-
Germ granules and gene regulation in the Caenorhabditis elegans germline.Genetics. 2022 Mar 3;220(3):iyab195. doi: 10.1093/genetics/iyab195. Genetics. 2022. PMID: 35239965 Free PMC article. Review.
-
Connecting the Dots: Linking Caenorhabditis elegans Small RNA Pathways and Germ Granules.Trends Cell Biol. 2021 May;31(5):387-401. doi: 10.1016/j.tcb.2020.12.012. Epub 2021 Jan 29. Trends Cell Biol. 2021. PMID: 33526340 Review.
Cited by
-
Down-regulation of tricarboxylic acid (TCA) cycle genes blocks progression through the first mitotic division in Caenorhabditis elegans embryos.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2602-7. doi: 10.1073/pnas.1311635111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24550289 Free PMC article.
-
Inhibition of mRNA maturation in trypanosomes causes the formation of novel foci at the nuclear periphery containing cytoplasmic regulators of mRNA fate.J Cell Sci. 2012 Jun 15;125(Pt 12):2896-909. doi: 10.1242/jcs.099275. Epub 2012 Feb 24. J Cell Sci. 2012. PMID: 22366449 Free PMC article.
-
Mechanisms of nuclear pore complex disassembly by the mitotic Polo-like kinase 1 (PLK-1) in C. elegans embryos.Sci Adv. 2023 Jul 21;9(29):eadf7826. doi: 10.1126/sciadv.adf7826. Epub 2023 Jul 19. Sci Adv. 2023. PMID: 37467327 Free PMC article.
-
New Family Members of FG Repeat Proteins and Their Unexplored Roles During Phase Separation.Front Cell Dev Biol. 2021 Jul 12;9:708702. doi: 10.3389/fcell.2021.708702. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34322491 Free PMC article. Review.
-
Nuclear envelope and genome interactions in cell fate.Front Genet. 2015 Mar 19;6:95. doi: 10.3389/fgene.2015.00095. eCollection 2015. Front Genet. 2015. PMID: 25852741 Free PMC article. Review.
References
-
- Ageberg M., Drott K., Olofsson T., Gullberg U., Lindmark A. (2008). Identification of a novel and myeloid specific role of the leukemia-associated fusion protein DEK-NUP214 leading to increased protein synthesis. Genes Chromosomes Cancer 47, 276-287 - PubMed
-
- Anderson P., Kedersha N. (2009). RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 10, 430-436 - PubMed
-
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., Gharakhani J., Julicher F., Hyman A. A. (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729-1732 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

