Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform
- PMID: 20333246
- PMCID: PMC2841623
- DOI: 10.1371/journal.pgen.1000884
Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform
Abstract
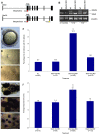
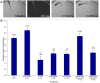
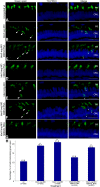
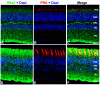
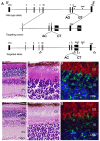
Bardet-Biedl Syndrome (BBS) is a heterogeneous syndromic form of retinal degeneration. We have identified a novel transcript of a known BBS gene, BBS3 (ARL6), which includes an additional exon. This transcript, BBS3L, is evolutionally conserved and is expressed predominantly in the eye, suggesting a specialized role in vision. Using antisense oligonucleotide knockdown in zebrafish, we previously demonstrated that bbs3 knockdown results in the cardinal features of BBS in zebrafish, including defects to the ciliated Kupffer's Vesicle and delayed retrograde melanosome transport. Unlike bbs3, knockdown of bbs3L does not result in Kupffer's Vesicle or melanosome transport defects, rather its knockdown leads to impaired visual function and mislocalization of the photopigment green cone opsin. Moreover, BBS3L RNA, but not BBS3 RNA, is sufficient to rescue both the vision defect as well as green opsin localization in the zebrafish retina. In order to demonstrate a role for Bbs3L function in the mammalian eye, we generated a Bbs3L-null mouse that presents with disruption of the normal photoreceptor architecture. Bbs3L-null mice lack key features of previously published Bbs-null mice, including obesity. These data demonstrate that the BBS3L transcript is required for proper retinal function and organization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Functional analysis of BBS3 A89V that results in non-syndromic retinal degeneration.Hum Mol Genet. 2011 Apr 15;20(8):1625-32. doi: 10.1093/hmg/ddr039. Epub 2011 Jan 31. Hum Mol Genet. 2011. PMID: 21282186 Free PMC article.
-
Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function.Hum Mol Genet. 2006 Mar 1;15(5):667-77. doi: 10.1093/hmg/ddi468. Epub 2006 Jan 6. Hum Mol Genet. 2006. PMID: 16399798
-
Genetic interaction between Bardet-Biedl syndrome genes and implications for limb patterning.Hum Mol Genet. 2008 Jul 1;17(13):1956-67. doi: 10.1093/hmg/ddn093. Epub 2008 Apr 1. Hum Mol Genet. 2008. PMID: 18381349 Free PMC article.
-
Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes.Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20678-83. doi: 10.1073/pnas.1113220108. Epub 2011 Dec 2. Proc Natl Acad Sci U S A. 2011. PMID: 22139371 Free PMC article.
-
Retinal dystrophy in Bardet-Biedl syndrome and related syndromic ciliopathies.Prog Retin Eye Res. 2011 Jul;30(4):258-74. doi: 10.1016/j.preteyeres.2011.03.001. Epub 2011 Apr 5. Prog Retin Eye Res. 2011. PMID: 21477661 Review.
Cited by
-
On the Wrong Track: Alterations of Ciliary Transport in Inherited Retinal Dystrophies.Front Cell Dev Biol. 2021 Mar 5;9:623734. doi: 10.3389/fcell.2021.623734. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748110 Free PMC article. Review.
-
Keeping an Eye on Bardet-Biedl Syndrome: A Comprehensive Review of the Role of Bardet-Biedl Syndrome Genes in the Eye.Med Res Arch. 2017 Sep;5(9):10.18103/mra.v5i9.1526. doi: 10.18103/mra.v5i9.1526. Epub 2017 Sep 18. Med Res Arch. 2017. PMID: 29457131 Free PMC article.
-
Mutations in a guanylate cyclase GCY-35/GCY-36 modify Bardet-Biedl syndrome-associated phenotypes in Caenorhabditis elegans.PLoS Genet. 2011 Oct;7(10):e1002335. doi: 10.1371/journal.pgen.1002335. Epub 2011 Oct 13. PLoS Genet. 2011. PMID: 22022287 Free PMC article.
-
Olfactory Loss and Dysfunction in Ciliopathies: Molecular Mechanisms and Potential Therapies.Curr Med Chem. 2019;26(17):3103-3119. doi: 10.2174/0929867325666180105102447. Curr Med Chem. 2019. PMID: 29303074 Free PMC article. Review.
-
Genetic architecture of retinal and macular degenerative diseases: the promise and challenges of next-generation sequencing.Genome Med. 2013 Oct 11;5(10):84. doi: 10.1186/gm488. eCollection 2013. Genome Med. 2013. PMID: 24112618 Free PMC article. Review.
References
-
- Green JS, Parfrey PS, Harnett JD, Farid NR, Cramer BC, et al. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med. 1989;321:1002–1009. - PubMed
-
- Harnett JD, Green JS, Cramer BC, Johnson G, Chafe L, et al. The spectrum of renal disease in Laurence-Moon-Biedl syndrome. N Engl J Med. 1988;319:615–618. - PubMed
-
- Bardet G. On congenital obesity syndrome with polydactyly and retinitis pigmentosa (a contribution to the study of clinical forms of hypophyseal obesity). 1920. Obes Res. 1995;3:387–399. - PubMed
-
- Biedl A. A pair of siblings with adiposo-genital dystrophy. 1922. Obes Res. 1995;3:404. - PubMed
-
- Elbedour K, Zucker N, Zalzstein E, Barki Y, Carmi R. Cardiac abnormalities in the Bardet-Biedl syndrome: echocardiographic studies of 22 patients. Am J Med Genet. 1994;52:164–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

