Activity of ceftaroline against recent emerging serotypes of Streptococcus pneumoniae in the United States
- PMID: 20308374
- PMCID: PMC2876371
- DOI: 10.1128/AAC.01797-09
Activity of ceftaroline against recent emerging serotypes of Streptococcus pneumoniae in the United States
Abstract
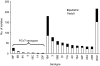
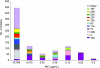
The in vitro activity of ceftaroline against 891 pneumococci collected in 2008 from 22 centers in the United States was investigated. Ceftaroline was the most potent agent tested, with the MICs being <0.008 to 0.5 microg/ml and the MIC(90)s being <0.008 to 0.25 microg/ml against 11 prevailing serotypes. The overall rates of susceptibility were as follows: penicillin G, 86.2%; ceftriaxone, 90.7%; cefuroxime, 70.1%; erythromycin, 61.6%; clindamycin, 79.2%; levofloxacin, 99.4%; and vancomycin, 100%. Serotype 19A isolates were the least susceptible. These results support the use of ceftaroline for the treatment of pneumococcal infections, including those caused by pneumococci resistant to other agents.
Figures
Similar articles
-
Decreased ceftriaxone susceptibility in emerging (35B and 6C) and persisting (19A) Streptococcus pneumoniae serotypes in the United States, 2011-2012: ceftaroline remains active in vitro among β-lactam agents.Antimicrob Agents Chemother. 2014 Aug;58(8):4923-7. doi: 10.1128/AAC.02976-14. Epub 2014 May 27. Antimicrob Agents Chemother. 2014. PMID: 24867974 Free PMC article.
-
In vitro evaluation of the antimicrobial activity of ceftaroline against cephalosporin-resistant isolates of Streptococcus pneumoniae.Antimicrob Agents Chemother. 2009 Feb;53(2):552-6. doi: 10.1128/AAC.01324-08. Epub 2008 Nov 17. Antimicrob Agents Chemother. 2009. PMID: 19015339 Free PMC article.
-
Ceftaroline Activity Against Multidrug-Resistant Streptococcus pneumoniae from U.S. Medical Centers (2014) and Molecular Characterization of a Single Ceftaroline Nonsusceptible Isolate.Microb Drug Resist. 2017 Jul;23(5):571-579. doi: 10.1089/mdr.2016.0258. Epub 2016 Dec 5. Microb Drug Resist. 2017. PMID: 27918694
-
In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008-2010).Clin Infect Dis. 2012 Sep;55 Suppl 3:S206-14. doi: 10.1093/cid/cis563. Clin Infect Dis. 2012. PMID: 22903953 Review.
-
Assessment of the activity of ceftaroline against clinical isolates of penicillin-intermediate and penicillin-resistant Streptococcus pneumoniae with elevated MICs of ceftaroline using an in vitro pharmacodynamic model.J Antimicrob Chemother. 2012 Jul;67(7):1706-11. doi: 10.1093/jac/dks113. Epub 2012 Mar 30. J Antimicrob Chemother. 2012. PMID: 22467630
Cited by
-
In vivo efficacy of ceftaroline fosamil in a methicillin-resistant panton-valentine leukocidin-producing Staphylococcus aureus rabbit pneumonia model.Antimicrob Agents Chemother. 2014;58(4):1855-61. doi: 10.1128/AAC.01707-13. Epub 2014 Jan 6. Antimicrob Agents Chemother. 2014. PMID: 24395236 Free PMC article.
-
Antimicrobial activity of ceftaroline tested against drug-resistant subsets of Streptococcus pneumoniae from U.S. medical centers.Antimicrob Agents Chemother. 2014;58(4):2468-71. doi: 10.1128/AAC.02557-13. Epub 2014 Feb 10. Antimicrob Agents Chemother. 2014. PMID: 24514082 Free PMC article.
-
In vitro activity of ceftaroline against gram-positive and gram-negative pathogens isolated from patients in Canadian hospitals in 2009.Antimicrob Agents Chemother. 2011 Jun;55(6):2837-46. doi: 10.1128/AAC.01787-10. Epub 2011 Mar 14. Antimicrob Agents Chemother. 2011. PMID: 21402844 Free PMC article.
-
Ceftaroline: A New Cephalosporin with Activity against Methicillin-Resistant Staphylococcus aureus (MRSA).Clin Med Rev Ther. 2011 Feb 10;3:a2466. doi: 10.4137/CMRT.S1637. Clin Med Rev Ther. 2011. PMID: 21785568 Free PMC article.
-
Critical appraisal of ceftaroline in the management of community-acquired bacterial pneumonia and skin infections.Ther Clin Risk Manag. 2012;8:149-56. doi: 10.2147/TCRM.S17413. Epub 2012 Mar 26. Ther Clin Risk Manag. 2012. PMID: 22547933 Free PMC article.
References
-
- Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376-1383. - PMC - PubMed
-
- Centers for Disease Control and Prevention. 2008. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998-2005. MMWR Morb. Mortal. Wkly. Rep. 57:144-148. - PubMed
-
- Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed. Approved standard M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- Corey, R., M. Wilcox, G. Talbot, T. Baculik, and D. Thye. 2008. CANVAS-1: randomized, double-blinded, phase 3 study (P903-06) of the efficacy and safety of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin structure infections (cSSSI), abstr. L-1515a. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical