Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study
- PMID: 20308317
- PMCID: PMC2940609
- DOI: 10.1093/neuonc/nop047
Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study
Abstract
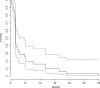
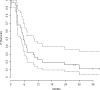
Immunostimulating oligodeoxynucleotides containing CpG motifs (CpG-ODN) have shown promising efficacy in cancer models when injected locally. In a phase I clinical trial, intratumoral infusions of CpG-ODN in glioblastoma (GBM) patients were well tolerated at doses up to 20 mg. This phase II trial was designed to study the efficacy of a local treatment by CpG-ODN in patients with recurrent GBMs. Patients with recurrent GBM occurring at least 3 months after radiotherapy, and previously treated with 1 or 2 regimens of chemotherapy received 20 mg of CpG-ODN (CpG-28) by convection-enhanced delivery. The primary endpoint was the percentage of patients without tumor progression 6 months after inclusion. Secondary endpoints were tolerance, survival, and radiological response. Thirty-four patients were enrolled in two centers between November 2004 and March 2006. Thirty-one patients received CpG-ODN treatment. The progression-free survival (PFS) at 6 months was 19%. One partial response and 3 minor responses were observed. The median overall survival was 28 weeks. Eight patients (24%) were alive 1 year after inclusion and 5 patients (15%) were alive after 2 years. Treatment was usually well tolerated. As reported previously, the most common toxicities were lymphopenia, mild fever, seizures, and transient neurological worsening. Despite a few cases showing a radiological response, CpG-28 showed modest activity on the 6-month PFS in this patient population. The molecular or clinical characteristics of a subgroup of patients that could potentially benefit from such an approach remain to be defined.
Figures
Similar articles
-
Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric, randomised study.Eur J Cancer. 2017 Mar;73:30-37. doi: 10.1016/j.ejca.2016.12.003. Epub 2017 Jan 28. Eur J Cancer. 2017. PMID: 28142059 Clinical Trial.
-
Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma.Neuro Oncol. 2006 Jan;8(1):60-6. doi: 10.1215/S1522851705000475. Neuro Oncol. 2006. PMID: 16443949 Free PMC article. Clinical Trial.
-
Clinical practice experience with NovoTTF-100A™ system for glioblastoma: The Patient Registry Dataset (PRiDe).Semin Oncol. 2014 Oct;41 Suppl 6:S4-S13. doi: 10.1053/j.seminoncol.2014.09.010. Epub 2014 Sep 16. Semin Oncol. 2014. PMID: 25213869
-
The Evolving Role of Tumor Treating Fields in Managing Glioblastoma: Guide for Oncologists.Am J Clin Oncol. 2018 Feb;41(2):191-196. doi: 10.1097/COC.0000000000000395. Am J Clin Oncol. 2018. PMID: 28832384 Free PMC article. Review.
-
Molecularly targeted therapies for recurrent glioblastoma: current and future targets.Neurosurg Focus. 2014 Dec;37(6):E15. doi: 10.3171/2014.9.FOCUS14519. Neurosurg Focus. 2014. PMID: 25434384 Free PMC article. Review.
Cited by
-
Convection-Enhanced Delivery in Malignant Gliomas: A Review of Toxicity and Efficacy.J Oncol. 2019 Jul 22;2019:9342796. doi: 10.1155/2019/9342796. eCollection 2019. J Oncol. 2019. PMID: 31428153 Free PMC article. Review.
-
Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity.Clin Cancer Res. 2011 Feb 15;17(4):771-82. doi: 10.1158/1078-0432.CCR-10-2444. Epub 2010 Nov 18. Clin Cancer Res. 2011. PMID: 21088258 Free PMC article.
-
Immunostimulatory CpG on Carbon Nanotubes Selectively Inhibits Migration of Brain Tumor Cells.Bioconjug Chem. 2018 May 16;29(5):1659-1668. doi: 10.1021/acs.bioconjchem.8b00146. Epub 2018 Apr 2. Bioconjug Chem. 2018. PMID: 29526082 Free PMC article.
-
Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier.Curr Pharm Des. 2016;22(9):1177-1193. doi: 10.2174/1381612822666151221150733. Curr Pharm Des. 2016. PMID: 26685681 Free PMC article. Review.
-
Convection-enhanced Delivery of Therapeutics for Malignant Gliomas.Neurol Med Chir (Tokyo). 2017 Jan 15;57(1):8-16. doi: 10.2176/nmc.ra.2016-0071. Epub 2016 Dec 15. Neurol Med Chir (Tokyo). 2017. PMID: 27980285 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Krieg AM. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6:88–95. - PubMed
-
- Takeshita F, Leifer CA, Gursel I, et al. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol. 2001;167:3555–3558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials