Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation
- PMID: 20299509
- PMCID: PMC2879099
- DOI: 10.1182/blood-2009-10-245001
Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation
Abstract
Although red blood cell (RBC) transfusions can be lifesaving, they are not without risk. In critically ill patients, RBC transfusions are associated with increased morbidity and mortality, which may increase with prolonged RBC storage before transfusion. The mechanisms responsible remain unknown. We hypothesized that acute clearance of a subset of damaged, stored RBCs delivers large amounts of iron to the monocyte/macrophage system, inducing inflammation. To test this in a well-controlled setting, we used a murine RBC storage and transfusion model to show that the transfusion of stored RBCs, or washed stored RBCs, increases plasma nontransferrin bound iron (NTBI), produces acute tissue iron deposition, and initiates inflammation. In contrast, the transfusion of fresh RBCs, or the infusion of stored RBC-derived supernatant, ghosts, or stroma-free lysate, does not produce these effects. Furthermore, the insult induced by transfusion of stored RBC synergizes with subclinical endotoxinemia producing clinically overt signs and symptoms. The increased plasma NTBI also enhances bacterial growth in vitro. Taken together, these results suggest that, in a mouse model, the cellular component of leukoreduced, stored RBC units contributes to the harmful effects of RBC transfusion that occur after prolonged storage. Nonetheless, these findings must be confirmed by prospective human studies.
Figures
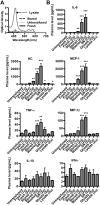

 ) or stored RBCs (n = 6; ■); *P < .01. (C) Circulating SAA1 protein levels in SAA1-luciferase reporter mice 24 hours after transfusion with fresh RBCs or stored RBCs (n = 6 per group); *P < .01. Results are combined from 2 separate experiments.
) or stored RBCs (n = 6; ■); *P < .01. (C) Circulating SAA1 protein levels in SAA1-luciferase reporter mice 24 hours after transfusion with fresh RBCs or stored RBCs (n = 6 per group); *P < .01. Results are combined from 2 separate experiments.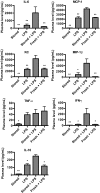
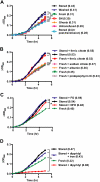
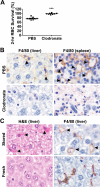
 ), the PBS vehicle control and stored RBCs (n = 3; ■), or 3 mg of DFO and stored RBCs (n = 6;
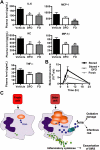
), the PBS vehicle control and stored RBCs (n = 3; ■), or 3 mg of DFO and stored RBCs (n = 6;  ); P = .095 at 4 and 6 hours after transfusion comparing vehicle-treated and DFO-treated mice. (C) Proposed mechanistic pathway (the “iron hypothesis”) explaining how transfusion of older stored RBCs may induce adverse effects in patients. Transfusion of stored, but not fresh, RBCs delivers an acute bolus of RBCs and RBC-derived iron to the monocyte/macrophage system resulting in oxidative stress and inflammatory cytokine secretion. Some of the macrophage-ingested iron is also released back into the circulation (ie, NTBI) where it can also cause oxidative damage and enhance bacterial proliferation. SIRS indicates systemic inflammatory response syndrome.
); P = .095 at 4 and 6 hours after transfusion comparing vehicle-treated and DFO-treated mice. (C) Proposed mechanistic pathway (the “iron hypothesis”) explaining how transfusion of older stored RBCs may induce adverse effects in patients. Transfusion of stored, but not fresh, RBCs delivers an acute bolus of RBCs and RBC-derived iron to the monocyte/macrophage system resulting in oxidative stress and inflammatory cytokine secretion. Some of the macrophage-ingested iron is also released back into the circulation (ie, NTBI) where it can also cause oxidative damage and enhance bacterial proliferation. SIRS indicates systemic inflammatory response syndrome.Similar articles
-
Harmful effects of transfusion of older stored red blood cells: iron and inflammation.Transfusion. 2011 Apr;51(4):881-5. doi: 10.1111/j.1537-2995.2011.03096.x. Transfusion. 2011. PMID: 21496050 Free PMC article.
-
Transfusion of 35-day stored red blood cells does not result in increase of plasma non-transferrin bound iron in human endotoxemia.Transfusion. 2017 Jan;57(1):53-59. doi: 10.1111/trf.13849. Epub 2016 Oct 3. Transfusion. 2017. PMID: 27696454 Clinical Trial.
-
Red blood cell transfusion is associated with increased hemolysis and an acute phase response in a subset of critically ill children.Am J Hematol. 2015 Oct;90(10):915-20. doi: 10.1002/ajh.24119. Am J Hematol. 2015. PMID: 26183122 Free PMC article. Clinical Trial.
-
Stored red blood cell transfusions: Iron, inflammation, immunity, and infection.Transfus Clin Biol. 2012 Jun;19(3):84-9. doi: 10.1016/j.tracli.2012.04.001. Epub 2012 Jun 7. Transfus Clin Biol. 2012. PMID: 22682673 Free PMC article. Review.
-
Clinical consequences of red cell storage in the critically ill.Transfusion. 2006 Nov;46(11):2014-27. doi: 10.1111/j.1537-2995.2006.01026.x. Transfusion. 2006. PMID: 17076859 Review.
Cited by
-
Neonatal transfusion models to determine the impact of using fresh red blood cells on inventory and exposure.Blood Transfus. 2015 Oct;13(4):595-9. doi: 10.2450/2015.0300-14. Epub 2015 Jun 12. Blood Transfus. 2015. PMID: 26192783 Free PMC article.
-
Quantifying Bone Marrow Fat Fraction and Iron by MRI for Distinguishing Aplastic Anemia from Myelodysplastic Syndromes.J Magn Reson Imaging. 2021 Dec;54(6):1754-1760. doi: 10.1002/jmri.27769. Epub 2021 Jun 11. J Magn Reson Imaging. 2021. PMID: 34117662 Free PMC article.
-
Ex-vivo expansion of red blood cells: how real for transfusion in humans?Blood Rev. 2012 Mar;26(2):81-95. doi: 10.1016/j.blre.2011.11.002. Epub 2011 Dec 15. Blood Rev. 2012. PMID: 22177597 Free PMC article. Review.
-
Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis.Arterioscler Thromb Vasc Biol. 2013 Aug;33(8):1861-71. doi: 10.1161/ATVBAHA.112.301068. Epub 2013 May 23. Arterioscler Thromb Vasc Biol. 2013. PMID: 23702660 Free PMC article.
-
Whole Blood Storage in CPDA1 Blood Bags Alters Erythrocyte Membrane Proteome.Oxid Med Cell Longev. 2018 Nov 8;2018:6375379. doi: 10.1155/2018/6375379. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30533175 Free PMC article.
References
-
- Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. - PubMed
-
- Whitaker B, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. Bethesda, MD: AABB; 2006.
-
- Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48(7):1478–1485. - PubMed
-
- Zeiler T, Muller JT, Kretschmer V. Flow-cytometric determination of survival time and 24-hour recovery of transfused red blood cells. Transfus Med Hemother. 2003;30:14–19.
-
- Ozment CP, Turi JL. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim Biophys Acta. 2009;1790(7):694–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

