IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis
- PMID: 20299353
- PMCID: PMC2900959
- DOI: 10.1681/ASN.2009060592
IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis
Abstract
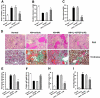
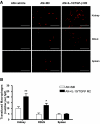
IL-10/TGF-beta-modified macrophages, a subset of activated macrophages, produce anti-inflammatory cytokines, suggesting that they may protect against inflammation-mediated injury. Here, macrophages modified ex vivo by IL-10/TGF-beta (IL-10/TGF-beta Mu2) significantly attenuated renal inflammation, structural injury, and functional decline in murine adriamycin nephrosis (AN). These cells deactivated effector macrophages and inhibited CD4+ T cell proliferation. IL-10/TGF-beta Mu2 expressed high levels of the regulatory co-stimulatory molecule B7-H4, induced regulatory T cells from CD4+CD25- T cells in vitro, and increased the number of regulatory T cells in lymph nodes draining the kidneys in AN. The phenotype of IL-10/TGF-beta Mu2 did not switch to that of effector macrophages in the inflamed kidney, and these cells did not promote fibrosis. Taken together, these data demonstrate that IL-10/TGF-beta-modified macrophages effectively protect against renal injury in AN and may become part of a therapeutic strategy for chronic inflammatory disease.
Figures
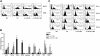

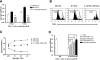

Similar articles
-
CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease.J Am Soc Nephrol. 2006 Oct;17(10):2731-41. doi: 10.1681/ASN.2005080842. Epub 2006 Sep 20. J Am Soc Nephrol. 2006. PMID: 16988067
-
Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease.Kidney Int. 2013 Oct;84(4):745-55. doi: 10.1038/ki.2013.135. Epub 2013 May 1. Kidney Int. 2013. PMID: 23636175
-
Transforming growth factor beta 1 costimulated growth and regulatory function of staphylococcal enterotoxin B-responsive CD8+ T cells.J Immunol. 1995 Jul 15;155(2):609-18. J Immunol. 1995. PMID: 7608539
-
Inhibition of human allergic T-helper type 2 immune responses by induced regulatory T cells requires the combination of interleukin-10-treated dendritic cells and transforming growth factor-beta for their induction.Clin Exp Allergy. 2006 Dec;36(12):1546-55. doi: 10.1111/j.1365-2222.2006.02601.x. Clin Exp Allergy. 2006. PMID: 17177678
-
Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease.Semin Immunol. 2004 Apr;16(2):135-43. doi: 10.1016/j.smim.2003.12.009. Semin Immunol. 2004. PMID: 15036237 Review.
Cited by
-
Adoptive transfer of immunomodulatory macrophages reduces the pro-inflammatory microenvironment and increases bone formation on titanium implants.Acta Biomater. 2024 Oct 15;188:432-445. doi: 10.1016/j.actbio.2024.09.011. Epub 2024 Sep 16. Acta Biomater. 2024. PMID: 39293568
-
IL-25 Elicits Innate Lymphoid Cells and Multipotent Progenitor Type 2 Cells That Reduce Renal Ischemic/Reperfusion Injury.J Am Soc Nephrol. 2015 Sep;26(9):2199-211. doi: 10.1681/ASN.2014050479. Epub 2015 Jan 2. J Am Soc Nephrol. 2015. PMID: 25556172 Free PMC article.
-
Increased Migratory and Activation Cell Markers of Peripheral Blood Lymphocytes in an Experimental Model of Nephrotic Syndrome.Mediators Inflamm. 2015;2015:209764. doi: 10.1155/2015/209764. Epub 2015 May 7. Mediators Inflamm. 2015. PMID: 26063968 Free PMC article.
-
The Impact of Versatile Macrophage Functions on Acute Kidney Injury and Its Outcomes.Front Physiol. 2019 Aug 6;10:1016. doi: 10.3389/fphys.2019.01016. eCollection 2019. Front Physiol. 2019. PMID: 31447703 Free PMC article.
-
HLA class II antibody activation of endothelial cells induces M2 macrophage differentiation in peripheral blood.Clin Exp Nephrol. 2023 Apr;27(4):309-320. doi: 10.1007/s10157-022-02307-9. Epub 2023 Jan 7. Clin Exp Nephrol. 2023. PMID: 36611129
References
-
- Gordon S, Taylor PR: Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. - PubMed
-
- Mosser DM: The many faces of macrophage activation. J Leukoc Biol 73: 209–212, 2003. - PubMed
-
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004. - PubMed
-
- Martinez FO, Sica A, Mantovani A, Locati M: Macrophage activation and polarization. Front Biosci 13: 453–461, 2008. - PubMed
-
- Atkins RC: Macrophages in renal injury. Am J Kidney Dis 31: xlv–xlvii, 1998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

