Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults
- PMID: 20238364
- PMCID: PMC6599830
- DOI: 10.1002/14651858.CD008272.pub2
Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults
Abstract
Background: According to consensus, initiation of therapy is best based on CD4 cell count, a marker of immune status, rather than on viral load, a marker of virologic replication. For patients with advanced symptoms, treatment should be started regardless of CD4 count. However, the point during the course of HIV infection at which antiretroviral therapy (ART) is best initiated in asymptomatic patients remains unclear. Guidelines issued by various agencies provide different initiation recommendations according to resource availability. This can be confusing for clinicians and policy-makers when determining the best time to initiate therapy. Optimizing the initiation of ART is clearly complex and must, therefore, be balanced between individual and broader public health needs.
Objectives: To assess the evidence for the optimal time to initiate ART in treatment-naive, asymptomatic, HIV-infected adults
Search strategy: We formulated a comprehensive and exhaustive search strategy in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). In August 2009, we searched the following electronic journal and trial databases: MEDLINE, EMBASE, and CENTRAL. We also searched the electronic conference database of NLM Gateway, individual conference proceedings and prospective trials registers. We contacted researchers and relevant organizations and checked reference lists of all included studies.
Selection criteria: Randomized controlled trials that compared the effect of ART consisting of three drugs initiated early in the disease at high CD4 counts as defined by the trial. Early initiation could be at levels of 201-350, 351-500, or >500 cells/microL, with the comparison group initiating ART at CD4 counts below 200 x 10(6) cells/microL or as defined by the trial.
Data collection and analysis: Two review authors independently assessed study eligibility, extracted data, and graded methodological quality. Data extraction and methodological quality were checked by a third author who resolved differences when these arose. Where clinically meaningful to do so, we meta-analysed dichotomous outcomes using the relative risk (RR) and report the 95% confidence intervals (95% CIs).
Main results: One completed trial (N = 816) and one sub-group (N = 249) of a larger trial met inclusion criteria. We combined the mortality data for both trials comparing initiating ART at CD4 levels at 350 cells/microL or between 200 and 350 cells/microL with deferring initiation of ART to CD4 levels of 250 cells/microL or 200 cells/microL. There was a statistically significant reduction in death when starting ART at higher CD4 counts. Risk of death was reduced by 74% (RR = 0.26; 95% CI: 0.11, 0.62; P = 0.002). Risk of tuberculosis was reduced by 50% in the groups starting ART early; this was not statistically significant, with the reduction as much as 74% or an increased risk of up to 12% (RR = 0.54; 95% CI: 0.26, 1.12; P = 0.01). Starting ART at enrollment (when participants had CD4 counts of 350 cells/microL) rather than deferring to starting at a CD4 count of 250 cells/microL reduced the risk of disease progression by 70%; this was not statistically significant, with the reduction in risk as much as 97% or an increased risk of up to 185% (RR = 0.30; 95% CI: 0.03, 2.85; P = 0.29).One RCT found no statistically significant difference in the number of independent Grade 3 or 4 adverse events occurring in the early and standard ART groups when we conducted an intention-to-treat analysis (RR = 1.72; 95% CI: 0.98, 3.03; P = 0.06). However, when analyzing only participants who actually commenced ART in the deferred group (n = 160), the trial authors report a statistically significant increase in the incidence of zidovudine-related anaemia (8.1%) compared with those in the early initiation group (3.4%) (RR = 0.42; 95% CI: 0.20, 0.88; P = 0.02).
Authors' conclusions: There is evidence of moderate quality that initiating ART at CD4 levels higher than 200 or 250 cells/microL reduces mortality rates in asymptomatic, ART-naive, HIV-infected people. Practitioners and policy-makers may consider initiating ART at levels </= 350 cells/microL for patients who present to health services and are diagnosed with HIV early in the infection.
Conflict of interest statement
None known
Figures
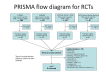
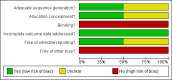
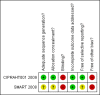





Update of
- doi: 10.1002/14651858.CD008272
Similar articles
-
Optimal monitoring strategies for guiding when to switch first-line antiretroviral therapy regimens for treatment failure in adults and adolescents living with HIV in low-resource settings.Cochrane Database Syst Rev. 2010 Apr 14;(4):CD008494. doi: 10.1002/14651858.CD008494. Cochrane Database Syst Rev. 2010. PMID: 20393969 Review.
-
Optimal time for initiating antiretroviral therapy (ART) in HIV-infected, treatment-naive children aged 2 to 5 years old.Cochrane Database Syst Rev. 2013 Oct 10;10(10):CD010309. doi: 10.1002/14651858.CD010309.pub2. Cochrane Database Syst Rev. 2013. PMID: 24114324 Free PMC article. Review.
-
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age.Cochrane Database Syst Rev. 2014 May 22;2014(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. Cochrane Database Syst Rev. 2014. PMID: 24852077 Free PMC article. Review.
-
Optimal timing for antiretroviral therapy initiation in patients with HIV infection and concurrent cryptococcal meningitis.Cochrane Database Syst Rev. 2013 Feb 28;(2):CD009012. doi: 10.1002/14651858.CD009012.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Jul 24;7:CD009012. doi: 10.1002/14651858.CD009012.pub3 PMID: 23450595 Updated. Review.
-
Efavirenz or nevirapine in three-drug combination therapy with two nucleoside or nucleotide-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2016 Dec 10;12(12):CD004246. doi: 10.1002/14651858.CD004246.pub4. Cochrane Database Syst Rev. 2016. PMID: 27943261 Free PMC article. Review.
Cited by
-
Factors associated with changes in uptake of HIV testing among young women (aged 15-24) in Tanzania from 2003 to 2012.Infect Dis Poverty. 2016 Sep 6;5(1):92. doi: 10.1186/s40249-016-0180-3. Infect Dis Poverty. 2016. PMID: 27595846 Free PMC article.
-
Perspectives of People Living with HIV on Access to Health Care: Protocol for a Scoping Review.JMIR Res Protoc. 2016 May 18;5(2):e71. doi: 10.2196/resprot.5263. JMIR Res Protoc. 2016. PMID: 27193076 Free PMC article.
-
Modelling HIV epidemics in the antiretroviral era: the UNAIDS Estimation and Projection package 2009.Sex Transm Infect. 2010 Dec;86 Suppl 2(Suppl_2):ii3-10. doi: 10.1136/sti.2010.044784. Epub 2010 Oct 6. Sex Transm Infect. 2010. PMID: 20929855 Free PMC article.
-
Universal testing and treatment as an HIV prevention strategy: research questions and methods.Curr HIV Res. 2011 Sep;9(6):429-45. doi: 10.2174/157016211798038515. Curr HIV Res. 2011. PMID: 21999778 Free PMC article. Review.
-
Late entry into HIV care: estimated impact on AIDS mortality rates in Brazil, 2003-2006.PLoS One. 2011 Jan 25;6(1):e14585. doi: 10.1371/journal.pone.0014585. PLoS One. 2011. PMID: 21283618 Free PMC article.
References
References to studies included in this review
CIPRAHT001 2009 {unpublished data only}
-
- Fitzgerald D. A randomized clinical trial of early versus strandard antiretroviral therapy for HIV‐infected patients with a CD4 T cell count of 200 ‐ 350 cells/ml (CIPRA HT 001). International AIDS Society Conference, Cape Town 2009.
SMART 2008 {published data only}
-
- El‐Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count‐guided interruption of antiretroviral treatment. The New England journal of medicine 2006;355(22):2283‐96. [PUBMED: 17135583] - PubMed
-
- Lundgren JD, Babiker A, El‐Sadr W, Emery S, Grund B, Neaton JD, et al. Inferior clinical outcome of the CD4+ cell count‐guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow‐up. The Journal of infectious diseases 2008;197(8):1145‐55. [PUBMED: 18476293] - PubMed
-
- The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. Major clinical outcomes in antiretroviral therapy (ART)‐naive participants and in those not receiving ART at baseline in the SMART study. The Journal of Infectious Diseases 2008;197(15 April):1133‐44. - PubMed
References to studies excluded from this review
Erhabor 2006 {published data only}
-
- Erhabor O, Ejele OA, Uko EK. HAART – Dependent CD4+ Lymphocyte Response Based onPre‐Therapeutic CD4 Lymphocyte Count in HIV‐Infected Nigerians. Annals of African Medicine 2006;5(3):153‐7.
References to ongoing studies
NCT00491556 {published data only}
-
- Early initiation of HAART. Ongoing study October 2007.
START 2009 {published data only}
-
- Strategic Timing of Antiretroviral Treatment (START). Ongoing study March 2009.
Additional references
Badri 2004
-
- Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, Wood R. Initiating highly active antiretroviral therapy in sub‐Saharan Africa: an assessment of the revised World Health Organization scaling‐up guidelines. AIDS (London, England) 2004;18(8):1159‐68. [PUBMED: 15166531] - PubMed
Braithwaite 2008
DAIDS 2009
-
- Division of AIDS. Table for grading the severity of Adult and Pediatric Adverse Events. Accessed 28 October 2009 http://rcc.tech‐res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GradingTable_Clarif....
Day 2002
-
- Day J, Brink B, Charalambous S, Churchyard G, Grant A, Morris D, et al. Clinical and operational guidelines for use of antiretroviral therapy in adults. Aurum Health Research 2002.
Deeks 2006
Erhabor 2006
-
- Erhabor O, Ejele OA, Uko EK. HAART ‐ Dependent CD4+ Lymphocyte Response Based on Pre‐Therapeutic CD4 Lymphocyte Count in HIV‐Infected Nigerians. Annals of African Medicine 2006;5(3):153‐57. [PUBMED: 17319344] - PubMed
Granich 2009
-
- Granich RM, Gilks CF, Dye C, Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009;373(9657):48‐57. - PubMed
Hammer 2008
-
- Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA, International AIDS Society‐USA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society‐USA panel. JAMA 2008;300(5):555‐70. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2008
-
- Higgens JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. [Available from www.cochrane‐handbook.org]
Kim 1987
-
- Kim K, DeMets DL. Design and analysis of group sequential tests based on the type 1 error spending rate function. Biometrika 1987;74:149‐54.
Kitahata 2009
Loubiere 2008
-
- Loubiere S, Filal KM, Sodqi M, Loundou A, Luchini S, Cleary S, et al. When to initiate highly active antiretroviral therapy in low‐resource settings: the Moroccan experience. Antiviral therapy 2008;13(2):241‐51. [PUBMED: 18505175] - PubMed
Moh 2007
-
- Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV‐infected adults in West Africa. AIDS (London, England) 2007;21(18):2483‐91. [PUBMED: 18025885] - PubMed
Montori 2005
-
- Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: a systematic review. JAMA 2005;294:2203‐09.. - PubMed
Orrell 2007
-
- Orrell C, Harling G, Lawn SD, Kaplan R, McNally M, Bekker L, Wood R. Conservation of first‐line antiretroviral treatmentregimen where therapeutic options are limited. Antiviral Therapy 2007;12. - PubMed
Palella 2003
-
- Palella FJ Jr, Deloria‐Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, Holmberg SD, HIV Outpatient Study Investigators. Survival benefit of initiating antiretroviral therapy in HIV‐infected persons in different CD4+ cell strata. Ann Intern Med 2003;138(8):620‐6.. - PubMed
Sabin 2009
-
- Sabin CA, Phillips AN. Should HIV therapy be started at a CD4 cell count above 350 cells/microl in asymptomatic HIV‐1‐infected patients?. Current opinion in infectious diseases 2009;22(2):191‐7. [PUBMED: 19283914] - PubMed
Schackman 2002
-
- Schackman BR, Freedberg KA, Weinstein MC, Sax PE, Losina E, Zhang H, Goldie SJ. Cost‐effectiveness implications of the timing of antiretroviral therapy in HIV‐infected adults. Arch Intern Med 2002;162(21):2478‐86. - PubMed
Schrader 2008
-
- Schrader S, Chuck SK, Rahn LW, Parekh P, Emrich KG. Significant improvements in self‐reported gastrointestinal tolerability, quality of life, patient satisfaction, and adherence with lopinavir/ritonavir tablet formulation compared with soft gel capsules. AIDS research and therapy 2008;5:21. [PUBMED: 18799008] - PMC - PubMed
Sterling 2001
-
- Sterling TR, Chaisson RE, Moore RD. HIV‐1 RNA, CD4 T‐lymphocytes, and clinical response to highly active antiretroviral therapy. AIDS (London, England) 2001;15(17):2251‐7. [PUBMED: 11698698] - PubMed
Sterne 2009
Walensky 2009
WHO 2006
-
- Charles Gilks, Marco Vitorio and the World Health Organisation guidelines development group. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach ‐ 2006 revision. http://www.who.int/hiv/pub/guidelines/adult/en/index.html (accessed July 2007).
WHO 2009
-
- World Health Organization. Rapid advice: Antiretroviral therapy for HIV infection in adults and adolescents. Geneva: World Health Organization November 2009.
Wong 2007
-
- Wong KH, Chan KC, Cheng KL, Chan WK, Kam KM, Lee SS. Establishing CD4 thresholds for highly active antiretroviral therapy initiation in a cohort of HIV‐infected adult Chinese in Hong Kong. AIDS patient care and STDs 2007;21(2):106‐15. [PUBMED: 17328660] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

