Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis
- PMID: 20233928
- PMCID: PMC2863568
- DOI: 10.1128/JB.00058-10
Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis
Abstract
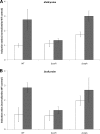
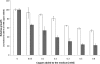
Copper and iron are essential elements for cellular growth. Although bacteria have to overcome limitations of these metals by affine and selective uptake, excessive amounts of both metals are toxic for the cells. Here we investigated the influences of copper stress on iron homeostasis in Bacillus subtilis, and we present evidence that copper excess leads to imbalances of intracellular iron metabolism by disturbing assembly of iron-sulfur cofactors. Connections between copper and iron homeostasis were initially observed in microarray studies showing upregulation of Fur-dependent genes under conditions of copper excess. This effect was found to be relieved in a csoR mutant showing constitutive copper efflux. In contrast, stronger Fur-dependent gene induction was found in a copper efflux-deficient copA mutant. A significant induction of the PerR regulon was not observed under copper stress, indicating that oxidative stress did not play a major role under these conditions. Intracellular iron and copper quantification revealed that the total iron content was stable during different states of copper excess or efflux and hence that global iron limitation did not account for copper-dependent Fur derepression. Strikingly, the microarray data for copper stress revealed a broad effect on the expression of genes coding for iron-sulfur cluster biogenesis (suf genes) and associated pathways such as cysteine biosynthesis and genes coding for iron-sulfur cluster proteins. Since these effects suggested an interaction of copper and iron-sulfur cluster maturation, a mutant with a conditional mutation of sufU, encoding the essential iron-sulfur scaffold protein in B. subtilis, was assayed for copper sensitivity, and its growth was found to be highly susceptible to copper stress. Further, different intracellular levels of SufU were found to influence the strength of Fur-dependent gene expression. By investigating the influence of copper on cluster-loaded SufU in vitro, Cu(I) was found to destabilize the scaffolded cluster at submicromolar concentrations. Thus, by interfering with iron-sulfur cluster formation, copper stress leads to enhanced expression of cluster scaffold and target proteins as well as iron and sulfur acquisition pathways, suggesting a possible feedback strategy to reestablish cluster biogenesis.
Figures
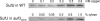
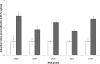
Similar articles
-
SufU is an essential iron-sulfur cluster scaffold protein in Bacillus subtilis.J Bacteriol. 2010 Mar;192(6):1643-51. doi: 10.1128/JB.01536-09. Epub 2010 Jan 22. J Bacteriol. 2010. PMID: 20097860 Free PMC article.
-
Distinct roles for U-type proteins in iron-sulfur cluster biosynthesis revealed by genetic analysis of the Bacillus subtilis sufCDSUB operon.Mol Microbiol. 2018 Mar;107(6):688-703. doi: 10.1111/mmi.13907. Epub 2018 Jan 18. Mol Microbiol. 2018. PMID: 29292548
-
Mechanistic characterization of sulfur transfer from cysteine desulfurase SufS to the iron-sulfur scaffold SufU in Bacillus subtilis.FEBS Lett. 2011 Feb 4;585(3):465-70. doi: 10.1016/j.febslet.2011.01.005. Epub 2011 Jan 12. FEBS Lett. 2011. PMID: 21236255
-
How Is Fe-S Cluster Formation Regulated?Annu Rev Microbiol. 2015;69:505-26. doi: 10.1146/annurev-micro-091014-104457. Annu Rev Microbiol. 2015. PMID: 26488283 Free PMC article. Review.
-
Fe-S cluster biogenesis by the bacterial Suf pathway.Biochim Biophys Acta Mol Cell Res. 2020 Nov;1867(11):118829. doi: 10.1016/j.bbamcr.2020.118829. Epub 2020 Aug 18. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32822728 Free PMC article. Review.
Cited by
-
Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload.Tuberculosis (Edinb). 2012 May;92(3):202-10. doi: 10.1016/j.tube.2011.12.006. Epub 2012 Feb 22. Tuberculosis (Edinb). 2012. PMID: 22361385 Free PMC article. Review.
-
Microbial Copper-binding Siderophores at the Host-Pathogen Interface.J Biol Chem. 2015 Jul 31;290(31):18967-74. doi: 10.1074/jbc.R115.644328. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055720 Free PMC article. Review.
-
Metal preferences and metallation.J Biol Chem. 2014 Oct 10;289(41):28095-103. doi: 10.1074/jbc.R114.588145. Epub 2014 Aug 26. J Biol Chem. 2014. PMID: 25160626 Free PMC article. Review.
-
Copper/Carbon Core/Shell Nanoparticles: A Potential Material to Control the Fish Pathogen Saprolegnia parasitica.Front Vet Sci. 2021 Jul 23;8:689085. doi: 10.3389/fvets.2021.689085. eCollection 2021. Front Vet Sci. 2021. PMID: 34368276 Free PMC article.
-
Correlated Transcriptional Responses Provide Insights into the Synergy Mechanisms of the Furazolidone, Vancomycin, and Sodium Deoxycholate Triple Combination in Escherichia coli.mSphere. 2021 Oct 27;6(5):e0062721. doi: 10.1128/mSphere.00627-21. Epub 2021 Sep 8. mSphere. 2021. PMID: 34494879 Free PMC article.
References
-
- Andreini, C., L. Banci, I. Bertini, and A. Rosato. 2008. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J. Proteome Res. 7:209-216. - PubMed
-
- Baichoo, N., T. Wang, R. Ye, and J. D. Helmann. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 45:1613-1629. - PubMed
-
- Baldi, P., and A. D. Long. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509-519. - PubMed
-
- Banci, L., I. Bertini, S. Ciofi-Baffoni, R. Del Conte, and L. Gonnelli. 2003. Understanding copper trafficking in bacteria: interaction between the copper transport protein CopZ and the N-terminal domain of the copper ATPase CopA from Bacillus subtilis. Biochemistry 42:1939-1949. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

