Effect of ceftaroline on normal human intestinal microflora
- PMID: 20231399
- PMCID: PMC2863678
- DOI: 10.1128/AAC.01716-09
Effect of ceftaroline on normal human intestinal microflora
Abstract
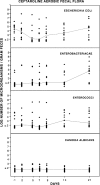
Ceftaroline is a new broad-spectrum cephalosporin being developed for the treatment of serious bacterial infections, including those caused by aerobic Gram-positive and Gram-negative bacteria. The purpose of the present study was to investigate the effect of administration of ceftaroline on the intestinal flora of healthy subjects. Twelve healthy subjects (6 males and 6 females), 20 to 41 years of age, received ceftaroline (600 mg) by intravenous infusion every 12 h (q12h) for 7 days. Plasma and feces were collected for determination of ceftaroline concentration and analysis of fecal flora. Fecal specimens were cultured on nonselective and selective media. Different colony types were counted, isolated in pure culture, and identified to the genus level. All new strains of colonizing bacteria were tested for susceptibility to ceftaroline. The concentrations of ceftaroline in plasma were as follows: on day 2, 17.5 to 34.8 mg/liter; on day 5, 19.7 to 33.2 mg/liter; and on day 7, 18.0 to 29.8 mg/liter. No ceftaroline concentrations were found on day -1, 9, 14, or 21. No measurable concentrations in feces were found on day -1, 2, 5, 7, 9, 14, or 21. There was a minor impact on the numbers of Escherichia coli strains, while the numbers of enterococci and Candida albicans strains were not affected. There were moderate decreases in the numbers of bifidobacteria and lactobacilli during the first 7 days, while the numbers of clostridia increased during the same period. No impact on the numbers of Bacteroides bacteria was noticed. No new colonizing aerobic or anaerobic bacteria resistant to ceftaroline (MIC >or= 4 mg/liter) were found. Ceftaroline had no significant ecological impact on the human intestinal microflora.
Figures

Similar articles
-
Effect of dalbavancin on the normal intestinal microflora.J Antimicrob Chemother. 2006 Sep;58(3):627-31. doi: 10.1093/jac/dkl281. Epub 2006 Jul 12. J Antimicrob Chemother. 2006. PMID: 16840427
-
Effect of ceftobiprole on the normal human intestinal microflora.Int J Antimicrob Agents. 2010 Dec;36(6):537-41. doi: 10.1016/j.ijantimicag.2010.07.021. Int J Antimicrob Agents. 2010. PMID: 20926263
-
Effect of tigecycline on normal oropharyngeal and intestinal microflora.Antimicrob Agents Chemother. 2006 Oct;50(10):3375-80. doi: 10.1128/AAC.00373-06. Antimicrob Agents Chemother. 2006. PMID: 17005820 Free PMC article.
-
Ceftaroline fosamil: a novel broad-spectrum cephalosporin with expanded anti-Gram-positive activity.J Antimicrob Chemother. 2010 Nov;65 Suppl 4:iv9-16. doi: 10.1093/jac/dkq251. J Antimicrob Chemother. 2010. PMID: 21115457 Review.
-
Ceftaroline: a new cephalosporin with activity against resistant gram-positive pathogens.Pharmacotherapy. 2010 Apr;30(4):375-89. doi: 10.1592/phco.30.4.375. Pharmacotherapy. 2010. PMID: 20334458 Review.
Cited by
-
Ceftaroline fosamil in the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.Drugs. 2012 Jul 30;72(11):1473-93. doi: 10.2165/11635660-000000000-00000. Drugs. 2012. PMID: 22779432 Review.
-
Gut Microbiota-brain Axis.Chin Med J (Engl). 2016 Oct 5;129(19):2373-80. doi: 10.4103/0366-6999.190667. Chin Med J (Engl). 2016. PMID: 27647198 Free PMC article. Review.
-
A Review of Combination Antimicrobial Therapy for Enterococcus faecalis Bloodstream Infections and Infective Endocarditis.Clin Infect Dis. 2018 Jul 2;67(2):303-309. doi: 10.1093/cid/ciy064. Clin Infect Dis. 2018. PMID: 29390132 Free PMC article. Review.
-
Treatment of Enterococcus faecalis Infective Endocarditis: A Continuing Challenge.Antibiotics (Basel). 2023 Apr 4;12(4):704. doi: 10.3390/antibiotics12040704. Antibiotics (Basel). 2023. PMID: 37107066 Free PMC article. Review.
-
Veterinary Medicine Needs New Green Antimicrobial Drugs.Front Microbiol. 2016 Aug 3;7:1196. doi: 10.3389/fmicb.2016.01196. eCollection 2016. Front Microbiol. 2016. PMID: 27536285 Free PMC article. Review.
References
-
- CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
-
- CLSI. 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 7th ed. Approved standard M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA. - PubMed
-
- Murray, P. R., E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.). 2007. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

