Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN
- PMID: 20228810
- PMCID: PMC3241509
- DOI: 10.1038/ncb2034
Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN
Erratum in
- Nat Cell Biol. 2010 May;12(5):520
Abstract
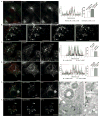
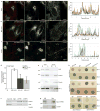
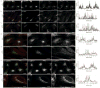
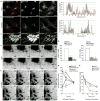
Actin dynamics is a tightly regulated process involved in various cellular events including biogenesis of clathrin-coated, AP-1 (adaptor protein 1)-coated transport carriers connecting the trans-Golgi network (TGN) and the endocytic pathway. However, the mechanisms coordinating coat assembly, membrane and actin remodelling during post-TGN transport remain poorly understood. Here we show that the Arf1 (ADP-ribosylation factor 1) GTPase synchronizes the TGN association of clathrin-AP-1 coats and protein complexes comprising CYFIP (cytoplasmic fragile-X mental retardation interacting protein; Sra, PIR121), a clathrin heavy chain binding protein associated with mental retardation. The Rac1 GTPase and its exchange factor beta-PIX (PAK-interacting exchange factor) activate these complexes, allowing N-WASP-dependent and Arp2/3-dependent actin polymerization towards membranes, thus promoting tubule formation. These phenomena can be recapitulated with synthetic membranes. This protein-network-based mechanism facilitates the sequential coordination of Arf1-dependent membrane priming, through the recruitment of coats and CYFIP-containing complexes, and of Rac1-dependent actin polymerization, and provides complementary but independent levels of regulation during early stages of clathrin-AP1-coated carrier biogenesis.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

Similar articles
-
Spatiotemporal Control of Lipid Conversion, Actin-Based Mechanical Forces, and Curvature Sensors during Clathrin/AP-1-Coated Vesicle Biogenesis.Cell Rep. 2017 Aug 29;20(9):2087-2099. doi: 10.1016/j.celrep.2017.08.013. Cell Rep. 2017. PMID: 28854360
-
Interaction of amphiphysins with AP-1 clathrin adaptors at the membrane.Biochem J. 2013 Feb 15;450(1):73-83. doi: 10.1042/BJ20121373. Biochem J. 2013. PMID: 23190214
-
Functional characterization of protein-sorting machineries at the trans-Golgi network in Drosophila melanogaster.J Cell Sci. 2010 Feb 1;123(Pt 3):460-71. doi: 10.1242/jcs.055103. Epub 2010 Jan 12. J Cell Sci. 2010. PMID: 20067992 Free PMC article.
-
A role for the small GTPase Rac1 in vaccinia actin-based motility.Small GTPases. 2015;6(2):119-22. doi: 10.1080/21541248.2015.1055182. Small GTPases. 2015. PMID: 26147090 Free PMC article. Review.
-
Clathrin and post-Golgi trafficking: a very complicated issue.Trends Plant Sci. 2014 Mar;19(3):134-9. doi: 10.1016/j.tplants.2013.10.008. Epub 2013 Nov 19. Trends Plant Sci. 2014. PMID: 24263003 Review.
Cited by
-
Cul3-KLHL20 ubiquitin ligase: physiological functions, stress responses, and disease implications.Cell Div. 2016 Apr 1;11:5. doi: 10.1186/s13008-016-0017-2. eCollection 2016. Cell Div. 2016. PMID: 27042198 Free PMC article. Review.
-
WAVE regulatory complex activation by cooperating GTPases Arf and Rac1.Proc Natl Acad Sci U S A. 2011 Aug 30;108(35):14449-54. doi: 10.1073/pnas.1107666108. Epub 2011 Aug 15. Proc Natl Acad Sci U S A. 2011. PMID: 21844371 Free PMC article.
-
Machine-Learning-Based Analysis in Genome-Edited Cells Reveals the Efficiency of Clathrin-Mediated Endocytosis.Cell Rep. 2015 Sep 29;12(12):2121-30. doi: 10.1016/j.celrep.2015.08.048. Epub 2015 Sep 17. Cell Rep. 2015. PMID: 26387943 Free PMC article.
-
OCRL1 engages with the F-BAR protein pacsin 2 to promote biogenesis of membrane-trafficking intermediates.Mol Biol Cell. 2016 Jan 1;27(1):90-107. doi: 10.1091/mbc.E15-06-0329. Epub 2015 Oct 28. Mol Biol Cell. 2016. PMID: 26510499 Free PMC article.
-
Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system.Nat Cell Biol. 2010 Sep;12(9):902-8. doi: 10.1038/ncb2094. Epub 2010 Aug 22. Nat Cell Biol. 2010. PMID: 20729836 Free PMC article.
References
-
- Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–12. - PubMed
-
- Edeling MA, Smith C, Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol. 2006;7:32–44. - PubMed
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–66. - PubMed
-
- Godi A, et al. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–7. - PubMed
-
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

