CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes
- PMID: 20228797
- PMCID: PMC2923071
- DOI: 10.1038/ni.1853
CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes
Abstract
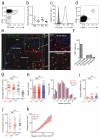
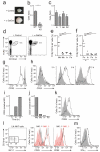
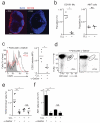
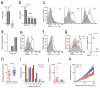
Invariant natural killer T cells (iNKT cells) are involved in the host defense against microbial infection. Although it is known that iNKT cells recognize glycolipids presented by CD1d, how and where they encounter antigen in vivo remains unclear. Here we used multiphoton microscopy to visualize the dynamics and activation of iNKT cells in lymph nodes. After antigen administration, iNKT cells became confined in a CD1d-dependent manner in close proximity to subcapsular sinus CD169(+) macrophages. These macrophages retained, internalized and presented lipid antigen and were required for iNKT cell activation, cytokine production and population expansion. Thus, CD169(+) macrophages can act as true antigen-presenting cells controlling early iNKT cell activation and favoring the fast initiation of immune responses.
Figures



Similar articles
-
Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation.Proc Natl Acad Sci U S A. 2013 May 7;110(19):7826-31. doi: 10.1073/pnas.1219888110. Epub 2013 Apr 22. Proc Natl Acad Sci U S A. 2013. PMID: 23610394 Free PMC article.
-
Border patrol: SCS macrophages activate iNKT cells too.Immunol Cell Biol. 2010 Aug;88(6):619-21. doi: 10.1038/icb.2010.71. Epub 2010 May 18. Immunol Cell Biol. 2010. PMID: 20479775 No abstract available.
-
Subcapsular sinus macrophage fragmentation and CD169+ bleb acquisition by closely associated IL-17-committed innate-like lymphocytes.PLoS One. 2012;7(6):e38258. doi: 10.1371/journal.pone.0038258. Epub 2012 Jun 1. PLoS One. 2012. PMID: 22675532 Free PMC article.
-
iNKT cells in microbial immunity: recognition of microbial glycolipids.Microbiol Immunol. 2011 Jul;55(7):472-82. doi: 10.1111/j.1348-0421.2011.00338.x. Microbiol Immunol. 2011. PMID: 21434991 Review.
-
Functions of CD1d-Restricted Invariant Natural Killer T Cells in Antimicrobial Immunity and Potential Applications for Infection Control.Front Immunol. 2018 Jun 6;9:1266. doi: 10.3389/fimmu.2018.01266. eCollection 2018. Front Immunol. 2018. PMID: 29928278 Free PMC article. Review.
Cited by
-
Sialoadhesin in recognition of self and non-self.Semin Immunopathol. 2012 May;34(3):353-64. doi: 10.1007/s00281-012-0310-3. Epub 2012 Mar 27. Semin Immunopathol. 2012. PMID: 22450957 Review.
-
Slam haplotypes modulate the response to lipopolysaccharide in vivo through control of NKT cell number and function.J Immunol. 2010 Jul 1;185(1):144-56. doi: 10.4049/jimmunol.0902658. Epub 2010 Jun 7. J Immunol. 2010. PMID: 20530260 Free PMC article.
-
Decision checkpoints in the thymus.Nat Immunol. 2010 Aug;11(8):666-73. doi: 10.1038/ni.1887. Epub 2010 Jul 20. Nat Immunol. 2010. PMID: 20644572 Free PMC article. Review.
-
Beyond CAR T Cells: Other Cell-Based Immunotherapeutic Strategies Against Cancer.Front Oncol. 2019 Apr 10;9:196. doi: 10.3389/fonc.2019.00196. eCollection 2019. Front Oncol. 2019. PMID: 31024832 Free PMC article. Review.
-
PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions.J Exp Med. 2011 Jun 6;208(6):1179-88. doi: 10.1084/jem.20102630. Epub 2011 May 30. J Exp Med. 2011. PMID: 21624939 Free PMC article.
References
-
- Cerundolo V, Silk J, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. - PubMed
-
- Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. - PubMed
-
- Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

