To build a synapse: signaling pathways in neuromuscular junction assembly
- PMID: 20215342
- PMCID: PMC2835321
- DOI: 10.1242/dev.038711
To build a synapse: signaling pathways in neuromuscular junction assembly
Abstract
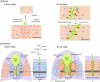
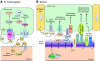
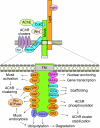
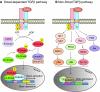
Synapses, as fundamental units of the neural circuitry, enable complex behaviors. The neuromuscular junction (NMJ) is a synapse type that forms between motoneurons and skeletal muscle fibers and that exhibits a high degree of subcellular specialization. Aided by genetic techniques and suitable animal models, studies in the past decade have brought significant progress in identifying NMJ components and assembly mechanisms. This review highlights recent advances in the study of NMJ development, focusing on signaling pathways that are activated by diffusible cues, which shed light on synaptogenesis in the brain and contribute to a better understanding of muscular dystrophy.
Figures





Similar articles
-
Glia cell line-derived neurotrophic factor regulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells.Neuroscience. 2004;128(3):497-509. doi: 10.1016/j.neuroscience.2004.06.067. Neuroscience. 2004. PMID: 15381279
-
The neuromuscular junction: selective remodeling of synaptic regulators at the nerve/muscle interface.Mech Dev. 2013 Jun-Aug;130(6-8):402-11. doi: 10.1016/j.mod.2012.09.004. Epub 2012 Sep 29. Mech Dev. 2013. PMID: 23032192 Review.
-
New dogs in the dogma: Lrp4 and Tid1 in neuromuscular synapse formation.Neuron. 2008 Nov 26;60(4):526-8. doi: 10.1016/j.neuron.2008.11.003. Neuron. 2008. PMID: 19038209 Review.
-
Molecular dissection of neuromuscular junction formation.Trends Neurosci. 2003 Jul;26(7):335-7. doi: 10.1016/S0166-2236(03)00131-0. Trends Neurosci. 2003. PMID: 12850425 Review.
-
LRP4 serves as a coreceptor of agrin.Neuron. 2008 Oct 23;60(2):285-97. doi: 10.1016/j.neuron.2008.10.006. Neuron. 2008. PMID: 18957220 Free PMC article.
Cited by
-
A role for the calmodulin kinase II-related anchoring protein (αkap) in maintaining the stability of nicotinic acetylcholine receptors.J Neurosci. 2012 Apr 11;32(15):5177-85. doi: 10.1523/JNEUROSCI.6477-11.2012. J Neurosci. 2012. PMID: 22496563 Free PMC article.
-
Slit2 as a β-catenin/Ctnnb1-dependent retrograde signal for presynaptic differentiation.Elife. 2015 Jul 10;4:e07266. doi: 10.7554/eLife.07266. Elife. 2015. PMID: 26159615 Free PMC article.
-
Assessment of the expression and role of the α1-nAChR subunit in efferent cholinergic function during the development of the mammalian cochlea.J Neurophysiol. 2016 Aug 1;116(2):479-92. doi: 10.1152/jn.01038.2015. Epub 2016 Apr 20. J Neurophysiol. 2016. PMID: 27098031 Free PMC article.
-
Serine residues in the α4 nicotinic acetylcholine receptor subunit regulate surface α4β2* receptor expression and clustering.Biochem Pharmacol. 2019 Jan;159:64-73. doi: 10.1016/j.bcp.2018.11.008. Epub 2018 Nov 9. Biochem Pharmacol. 2019. PMID: 30414940 Free PMC article.
-
Anterograde Jelly belly ligand to Alk receptor signaling at developing synapses is regulated by Mind the gap.Development. 2010 Oct;137(20):3523-33. doi: 10.1242/dev.047878. Development. 2010. PMID: 20876658 Free PMC article.
References
-
- Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhaes T. R., Goodman C. S. (2002). wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545-558 - PubMed
-
- Akaaboune M., Culican S. M., Turney S. G., Lichtman J. W. (1999). Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science 286, 503-557 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

