The Golgi and the centrosome: building a functional partnership
- PMID: 20212314
- PMCID: PMC2835931
- DOI: 10.1083/jcb.200910001
The Golgi and the centrosome: building a functional partnership
Abstract
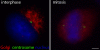
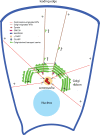
The mammalian Golgi apparatus is characterized by a ribbon-like organization adjacent to the centrosome during interphase and extensive fragmentation and dispersal away from the centrosome during mitosis. It is not clear whether this dynamic association between the Golgi and centrosome is of functional significance. We discuss recent findings indicating that the Golgi-centrosome relationship may be important for directional protein transport and centrosome positioning, which are both required for cell polarization. We also summarize our current knowledge of the link between Golgi organization and cell cycle progression.
Figures

Similar articles
-
Functional Coordination among the Golgi Complex, the Centrosome and the Microtubule Cytoskeleton during the Cell Cycle.Cells. 2022 Jan 21;11(3):354. doi: 10.3390/cells11030354. Cells. 2022. PMID: 35159164 Free PMC article. Review.
-
Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis.J Cell Biol. 2011 May 30;193(5):917-33. doi: 10.1083/jcb.201011014. Epub 2011 May 23. J Cell Biol. 2011. PMID: 21606206 Free PMC article.
-
The Golgi protein GM130 regulates centrosome morphology and function.Mol Biol Cell. 2008 Feb;19(2):745-53. doi: 10.1091/mbc.e07-08-0847. Epub 2007 Nov 28. Mol Biol Cell. 2008. PMID: 18045989 Free PMC article.
-
Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells.Nat Cell Biol. 2009 Sep;11(9):1069-80. doi: 10.1038/ncb1920. Epub 2009 Aug 23. Nat Cell Biol. 2009. PMID: 19701196 Free PMC article.
-
Positioning centrosomes and spindle poles: looking at the periphery to find the centre.Biol Cell. 2006 Sep;98(9):557-65. doi: 10.1042/BC20060017. Biol Cell. 2006. PMID: 16907664 Review.
Cited by
-
Ror2 enhances polarity and directional migration of primordial germ cells.PLoS Genet. 2011 Dec;7(12):e1002428. doi: 10.1371/journal.pgen.1002428. Epub 2011 Dec 22. PLoS Genet. 2011. PMID: 22216013 Free PMC article.
-
Actin-driven Golgi apparatus dispersal during collective migration of epithelial cells.Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2204808119. doi: 10.1073/pnas.2204808119. Epub 2022 Jun 24. Proc Natl Acad Sci U S A. 2022. PMID: 35749357 Free PMC article.
-
BPAG1a and b associate with EB1 and EB3 and modulate vesicular transport, Golgi apparatus structure, and cell migration in C2.7 myoblasts.PLoS One. 2014 Sep 22;9(9):e107535. doi: 10.1371/journal.pone.0107535. eCollection 2014. PLoS One. 2014. PMID: 25244344 Free PMC article.
-
Schizophrenia-associated Mitotic Arrest Deficient-1 (MAD1) regulates the polarity of migrating neurons in the developing neocortex.Mol Psychiatry. 2023 Feb;28(2):856-870. doi: 10.1038/s41380-022-01856-5. Epub 2022 Nov 10. Mol Psychiatry. 2023. PMID: 36357673 Free PMC article.
-
Cell polarization: From epithelial cells to odontoblasts.Eur J Cell Biol. 2019 Jan;98(1):1-11. doi: 10.1016/j.ejcb.2018.11.003. Epub 2018 Nov 17. Eur J Cell Biol. 2019. PMID: 30473389 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

