Genomic imprinting of experience-dependent cortical plasticity by the ubiquitin ligase gene Ube3a
- PMID: 20212164
- PMCID: PMC2851788
- DOI: 10.1073/pnas.1001281107
Genomic imprinting of experience-dependent cortical plasticity by the ubiquitin ligase gene Ube3a
Abstract
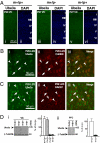
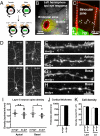
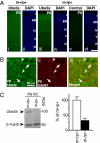
A defect in the maternal copy of a ubiqutin ligase gene Ube3a can produce a neurodevelopmental defect in human children known as Angelman syndrome. We investigated the role of the maternally expressed Ube3a gene in experience-dependent development and plasticity of the mouse visual system. As demonstrated by optical imaging, rapid ocular dominance (OD) plasticity after brief monocular deprivation (MD) was severely impaired during the critical period (CP) in the visual cortex (VC) of Ube3a maternal-deficient (m-/p+) mice. Prolonged MD elicited significant plasticity in m-/p+ mice that never matched the level seen in control animals. In older animals after the CP, 7-day MD elicited mild OD shifts in both control and m-/p+ mice; however, the OD shifts in m-/p+ mice lacked the strengthening of visual responses to the two eyes characteristic of normal adult plasticity. Anatomic effects of the maternal deficiency include reduced spine density on basal, but not apical, dendrites of pyramidal neurons in the binocular region of the VC. Imprinting of Ube3a expression was not fully established in the early postnatal period, consistent with the normal development of cortical retinotopy and visual acuity that we observed in m-/p+ mice, but was fully established by the onset of the CP. These results demonstrate that paternal and maternal genomes are not functionally equivalent for cortical plasticity, and that maternally expressed Ube3a is required for normal experience-dependent modification of cortical circuits during and after the CP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Maternal Loss of Ube3a Impairs Experience-Driven Dendritic Spine Maintenance in the Developing Visual Cortex.J Neurosci. 2016 Apr 27;36(17):4888-94. doi: 10.1523/JNEUROSCI.4204-15.2016. J Neurosci. 2016. PMID: 27122043 Free PMC article.
-
Ube3a is required for experience-dependent maturation of the neocortex.Nat Neurosci. 2009 Jun;12(6):777-83. doi: 10.1038/nn.2327. Epub 2009 May 10. Nat Neurosci. 2009. PMID: 19430469 Free PMC article.
-
The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology.Hum Mol Genet. 2008 Jan 1;17(1):111-8. doi: 10.1093/hmg/ddm288. Epub 2007 Oct 16. Hum Mol Genet. 2008. PMID: 17940072
-
Potential therapeutic approaches for Angelman syndrome.Expert Opin Ther Targets. 2016;20(5):601-13. doi: 10.1517/14728222.2016.1115837. Epub 2015 Nov 26. Expert Opin Ther Targets. 2016. PMID: 26558806 Free PMC article. Review.
-
Angelman syndrome - insights into a rare neurogenetic disorder.Nat Rev Neurol. 2016 Oct;12(10):584-93. doi: 10.1038/nrneurol.2016.133. Epub 2016 Sep 12. Nat Rev Neurol. 2016. PMID: 27615419 Review.
Cited by
-
Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities.Cold Spring Harb Perspect Biol. 2012 Mar 1;4(3):a009886. doi: 10.1101/cshperspect.a009886. Cold Spring Harb Perspect Biol. 2012. PMID: 22258914 Free PMC article. Review.
-
Environmental Enrichment Improves Behavioral Abnormalities in a Mouse Model of Angelman Syndrome.Mol Neurobiol. 2017 Sep;54(7):5319-5326. doi: 10.1007/s12035-016-0080-3. Epub 2016 Sep 1. Mol Neurobiol. 2017. PMID: 27581300
-
Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice.Neuron. 2011 Apr 14;70(1):35-42. doi: 10.1016/j.neuron.2011.03.001. Neuron. 2011. PMID: 21482354 Free PMC article.
-
The active form of E6-associated protein (E6AP)/UBE3A ubiquitin ligase is an oligomer.J Biol Chem. 2014 Jan 10;289(2):1033-48. doi: 10.1074/jbc.M113.517805. Epub 2013 Nov 22. J Biol Chem. 2014. PMID: 24273172 Free PMC article.
-
Experience-Dependent Changes in Myelin Basic Protein Expression in Adult Visual and Somatosensory Cortex.Front Cell Neurosci. 2020 Mar 17;14:56. doi: 10.3389/fncel.2020.00056. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32265660 Free PMC article.
References
-
- Wilkinson LS, Davies W, Isles AR. Genomic imprinting effects on brain development and function. Nat Rev Neurosci. 2007;8:832–843. - PubMed
-
- McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–183. - PubMed
-
- Barton SC, Surani MA, Norris ML. Role of paternal and maternal genomes in mouse development. Nature. 1984;311:374–376. - PubMed
-
- Keverne EB, Fundele R, Narasimha M, Barton SC, Surani MA. Genomic imprinting and the differential roles of parental genomes in brain development. Brain Res Dev Brain Res. 1996;92:91–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

