IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection
- PMID: 20212069
- PMCID: PMC2839152
- DOI: 10.1084/jem.20091711
IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection
Abstract
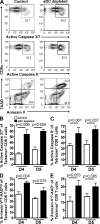
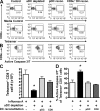
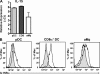
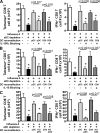
We have recently demonstrated that peripheral CD8 T cells require two separate activation hits to accumulate to high numbers in the lungs after influenza virus infection: a primary interaction with mature, antigen-bearing dendritic cells (DCs) in the lymph node, and a second, previously unrecognized interaction with MHC I-viral antigen-bearing pulmonary DCs in the lungs. We demonstrate that in the absence of lung-resident DC subsets, virus-specific CD8 T cells undergo significantly increased levels of apoptosis in the lungs; however, reconstitution with pulmonary plasmacytoid DCs and CD8alpha(+) DCs promotes increased T cell survival and accumulation in the lungs. Further, our results show that the absence of DCs after influenza virus infection results in significantly reduced levels of IL-15 in the lungs and that pulmonary DC-mediated rescue of virus-specific CD8 T cell responses in the lungs requires trans-presentation of IL-15 via DC-expressed IL-15Ralpha. This study demonstrates a key, novel requirement for DC trans-presented IL-15 in promoting effector CD8 T cell survival in the respiratory tract after virus infection, and suggests that this trans-presentation could be an important target for the development of unique antiviral therapies and more effective vaccine strategies.
Figures




Similar articles
-
Plasmacytoid Dendritic Cells Require Direct Infection To Sustain the Pulmonary Influenza A Virus-Specific CD8 T Cell Response.J Virol. 2015 Dec 30;90(6):2830-7. doi: 10.1128/JVI.02546-15. J Virol. 2015. PMID: 26719269 Free PMC article.
-
Dendritic cells and CD28 costimulation are required to sustain virus-specific CD8+ T cell responses during the effector phase in vivo.J Immunol. 2011 Apr 15;186(8):4599-608. doi: 10.4049/jimmunol.1001972. Epub 2011 Mar 9. J Immunol. 2011. PMID: 21389258
-
Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells.J Immunol. 2011 Dec 1;187(11):6011-21. doi: 10.4049/jimmunol.1100987. Epub 2011 Oct 31. J Immunol. 2011. PMID: 22043017
-
Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens.Immunol Lett. 2007 Dec 15;114(2):66-72. doi: 10.1016/j.imlet.2007.09.007. Epub 2007 Oct 12. Immunol Lett. 2007. PMID: 17964665 Review.
-
CD8 T cell responses to influenza virus infection in aged mice.Ageing Res Rev. 2011 Sep;10(4):422-7. doi: 10.1016/j.arr.2011.02.001. Epub 2011 Feb 15. Ageing Res Rev. 2011. PMID: 21315186 Free PMC article. Review.
Cited by
-
Molecular mechanisms involved in dendritic cell dysfunction in cancer.Cell Mol Life Sci. 2017 Mar;74(5):761-776. doi: 10.1007/s00018-016-2317-8. Epub 2016 Aug 5. Cell Mol Life Sci. 2017. PMID: 27491428 Free PMC article. Review.
-
Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti-viral CD8(+) T cells.PLoS One. 2012;7(10):e46581. doi: 10.1371/journal.pone.0046581. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056353 Free PMC article.
-
Malignant and Benign T Cells Constituting Cutaneous T-Cell Lymphoma.Int J Mol Sci. 2021 Nov 29;22(23):12933. doi: 10.3390/ijms222312933. Int J Mol Sci. 2021. PMID: 34884736 Free PMC article. Review.
-
T cell responses during influenza infection: getting and keeping control.Trends Immunol. 2011 May;32(5):225-31. doi: 10.1016/j.it.2011.02.006. Epub 2011 Mar 23. Trends Immunol. 2011. PMID: 21435950 Free PMC article. Review.
-
Diverse Epitope Specificity, Immunodominance Hierarchy, and Functional Avidity of Effector CD4 T Cells Established During Priming Is Maintained in Lung After Influenza A Virus Infection.Front Immunol. 2018 Apr 6;9:655. doi: 10.3389/fimmu.2018.00655. eCollection 2018. Front Immunol. 2018. PMID: 29681900 Free PMC article.
References
-
- Akbar A.N., Borthwick N.J., Wickremasinghe R.G., Panayoitidis P., Pilling D., Bofill M., Krajewski S., Reed J.C., Salmon M. 1996. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 26:294–299 10.1002/eji.1830260204 - DOI - PubMed
-
- Aldridge J.R., Jr., Moseley C.E., Boltz D.A., Negovetich N.J., Reynolds C., Franks J., Brown S.A., Doherty P.C., Webster R.G., Thomas P.G. 2009. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. USA. 106:5306–5311 10.1073/pnas.0900655106 - DOI - PMC - PubMed
-
- Bansal-Pakala P., Halteman B.S., Cheng M.H., Croft M. 2004. Costimulation of CD8 T cell responses by OX40. J. Immunol. 172:4821–4825 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

