Ush1c gene expression levels in the ear and eye suggest different roles for Ush1c in neurosensory organs in a new Ush1c knockout mouse
- PMID: 20211154
- PMCID: PMC2907663
- DOI: 10.1016/j.brainres.2010.02.079
Ush1c gene expression levels in the ear and eye suggest different roles for Ush1c in neurosensory organs in a new Ush1c knockout mouse
Abstract
Usher syndrome (USH) is the most common form of deaf-blindness in humans. Molecular characterization revealed that the USH gene products form a macromolecular protein network in hair cells of the inner ear and in photoreceptor cells of the retina via binding to PDZ domains in the scaffold protein harmonin encoded by the Ush1c gene in mice and humans. Although several mouse mutants for the Ush1c gene have been described, we generated a targeted null mutation Ush1c mouse model in which the first four exons of the Ush1c gene were replaced with a reporter gene. Here, we assessed the expression pattern of the reporter gene under control of Ush1c regulatory elements and characterized the phenotype of mice defective for Ush1c. These Ush1 knockout mice are deaf but do not recapitulate vision defects before 10 months of age. Our data show LacZ expression in multiple layers of the retina but in neither outer nor inner segments of the photoreceptor layers in mice bearing the knockout construct at 1-5 months of age. The fact that Ush1c expression is much higher in the ear than in the eye suggests a different role for Ush1c in ear function than in the eye and may explain why Ush1c mutant mice do not recapitulate vision defects.
Published by Elsevier B.V.
Figures


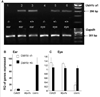
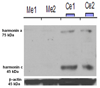



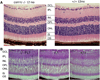
Similar articles
-
Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease.Exp Eye Res. 2006 Jul;83(1):97-119. doi: 10.1016/j.exer.2005.11.010. Epub 2006 Mar 20. Exp Eye Res. 2006. PMID: 16545802 Review.
-
Analysis of subcellular localization of Myo7a, Pcdh15 and Sans in Ush1c knockout mice.Int J Exp Pathol. 2011 Feb;92(1):66-71. doi: 10.1111/j.1365-2613.2010.00751.x. Epub 2010 Dec 13. Int J Exp Pathol. 2011. PMID: 21156003 Free PMC article.
-
Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2.Hum Mol Genet. 2005 Dec 15;14(24):3933-43. doi: 10.1093/hmg/ddi417. Epub 2005 Nov 21. Hum Mol Genet. 2005. PMID: 16301216
-
Photoreceptor expression of the Usher syndrome type 1 protein protocadherin 15 (USH1F) and its interaction with the scaffold protein harmonin (USH1C).Mol Vis. 2005 May 12;11:347-55. Mol Vis. 2005. PMID: 15928608
-
Usher syndrome protein network functions in the retina and their relation to other retinal ciliopathies.Adv Exp Med Biol. 2014;801:527-33. doi: 10.1007/978-1-4614-3209-8_67. Adv Exp Med Biol. 2014. PMID: 24664740 Review.
Cited by
-
Transcriptomic Analyses of Inner Ear Sensory Epithelia in Zebrafish.Anat Rec (Hoboken). 2020 Mar;303(3):527-543. doi: 10.1002/ar.24331. Epub 2019 Dec 28. Anat Rec (Hoboken). 2020. PMID: 31883312 Free PMC article.
-
Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion.Cell. 2014 Apr 10;157(2):433-446. doi: 10.1016/j.cell.2014.01.067. Cell. 2014. PMID: 24725409 Free PMC article.
-
The Time Course of Deafness and Retinal Degeneration in a Kunming Mouse Model for Usher Syndrome.PLoS One. 2016 May 17;11(5):e0155619. doi: 10.1371/journal.pone.0155619. eCollection 2016. PLoS One. 2016. PMID: 27186975 Free PMC article.
-
Single-cell transcriptomic profiling of the mouse cochlea: An atlas for targeted therapies.Proc Natl Acad Sci U S A. 2023 Jun 27;120(26):e2221744120. doi: 10.1073/pnas.2221744120. Epub 2023 Jun 20. Proc Natl Acad Sci U S A. 2023. PMID: 37339214 Free PMC article.
-
Usher Syndrome: Genetics of a Human Ciliopathy.Int J Mol Sci. 2021 Jun 23;22(13):6723. doi: 10.3390/ijms22136723. Int J Mol Sci. 2021. PMID: 34201633 Free PMC article. Review.
References
-
- Ahmed ZM, Riazuddin S, Riazuddin S, Wilcox ER. The molecular genetics of usher syndrome. Clin Genet. 2003;63:431–444. - PubMed
-
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse ames waltzer hearing-loss mutant is caused by mutation of pcdh15, a novel protocadherin gene. Nat Genet. 2001a;27:9–102. - PubMed
-
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, Schwartz S, Lee C, Morton CC, Mullins RF, Ramesh A, Van Camp G, Hageman GS, Woychik RP, Smith RJ. Mutations in the novel protocadherin pcdh15 cause usher syndrome type 1f. Hum Mol Genet. 2001b;10:1709–1718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC012115/DC/NIDCD NIH HHS/United States
- R01 DC007392-01A1/DC/NIDCD NIH HHS/United States
- DC05575/DC/NIDCD NIH HHS/United States
- DC004301/DC/NIDCD NIH HHS/United States
- DC009246/DC/NIDCD NIH HHS/United States
- R03 DC004376-03/DC/NIDCD NIH HHS/United States
- P30 CA043703/CA/NCI NIH HHS/United States
- R01 DC009246/DC/NIDCD NIH HHS/United States
- R01 DC009246-01A2/DC/NIDCD NIH HHS/United States
- R21 DC005846-04/DC/NIDCD NIH HHS/United States
- R01 DC005575/DC/NIDCD NIH HHS/United States
- R01 DC007392-02/DC/NIDCD NIH HHS/United States
- R21 DC005846-03/DC/NIDCD NIH HHS/United States
- R21 DC005846/DC/NIDCD NIH HHS/United States
- R01 DC007392-04/DC/NIDCD NIH HHS/United States
- R01 DC007392/DC/NIDCD NIH HHS/United States
- P30 CA43703/CA/NCI NIH HHS/United States
- DC007392/DC/NIDCD NIH HHS/United States
- R01 DC004301/DC/NIDCD NIH HHS/United States
- R01 DC007392-03/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

