IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis
- PMID: 20200277
- PMCID: PMC2914630
- DOI: 10.4049/jimmunol.0903151
IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis
Abstract
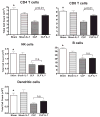
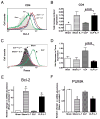
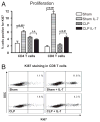
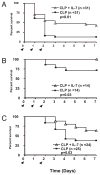
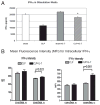
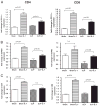
Sepsis is a highly lethal disorder characterized by widespread apoptosis-induced depletion of immune cells and the development of a profound immunosuppressive state. IL-7 is a potent antiapoptotic cytokine that enhances immune effector cell function and is essential for lymphocyte survival. In this study, recombinant human IL-7 (rhIL-7) efficacy and potential mechanisms of action were tested in a murine peritonitis model. Studies at two independent laboratories showed that rhIL-7 markedly improved host survival, blocked apoptosis of CD4 and CD8 T cells, restored IFN-gamma production, and improved immune effector cell recruitment to the infected site. Importantly, rhIL-7 also prevented a hallmark of sepsis (i.e., the loss of delayed-type hypersensitivity), which is an IFN-gamma- and T cell-dependent response. Mechanistically, rhIL-7 significantly increased the expression of the leukocyte adhesion markers LFA-1 and VLA-4, consistent with its ability to improve leukocyte function and trafficking to the infectious focus. rhIL-7 also increased the expression of CD8. The potent antiapoptotic effect of rhIL-7 was due to increased Bcl-2, as well as to a dramatic decrease in sepsis-induced PUMA, a heretofore unreported effect of IL-7. If additional animal studies support its efficacy in sepsis and if current clinical trials continue to confirm its safety in diverse settings, rhIL-7 should be strongly considered for clinical trials in sepsis.
Conflict of interest statement
Dr. Michel Morre is the CEO of Cytheris Corporation, a company that makes IL-7 for clinical trials. The other authors have no financial conflicts of interest.
Figures
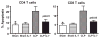

Comment in
-
Comment on "IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis" and comment on "IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis".J Immunol. 2010 Jul 15;185(2):789; author reply 789-90. doi: 10.4049/jimmunol.1090052. J Immunol. 2010. PMID: 20601609 No abstract available.
Similar articles
-
Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of Pseudomonas aeruginosa pneumonia.J Leukoc Biol. 2017 Feb;101(2):543-554. doi: 10.1189/jlb.4A1215-581R. Epub 2016 Sep 14. J Leukoc Biol. 2017. PMID: 27630218 Free PMC article.
-
Comment on "IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis" and comment on "IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis".J Immunol. 2010 Jul 15;185(2):789; author reply 789-90. doi: 10.4049/jimmunol.1090052. J Immunol. 2010. PMID: 20601609 No abstract available.
-
IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis.J Immunol. 2010 Feb 1;184(3):1401-9. doi: 10.4049/jimmunol.0902307. Epub 2009 Dec 21. J Immunol. 2010. PMID: 20026737 Free PMC article.
-
IL-7 and Its Beneficial Role in Sepsis-Induced T Lymphocyte Dysfunction.Crit Rev Immunol. 2018;38(6):433-451. doi: 10.1615/CritRevImmunol.2018027460. Crit Rev Immunol. 2018. PMID: 31002599 Review.
-
New therapies for sepsis: focus on the interleukin (IL)12 family member IL27.Ann Rheum Dis. 2007 Nov;66 Suppl 3(Suppl 3):iii29-31. doi: 10.1136/ard.2007.078337. Ann Rheum Dis. 2007. PMID: 17934090 Free PMC article. Review.
Cited by
-
Navigating the Modern Landscape of Sepsis: Advances in Diagnosis and Treatment.Int J Mol Sci. 2024 Jul 5;25(13):7396. doi: 10.3390/ijms25137396. Int J Mol Sci. 2024. PMID: 39000503 Free PMC article. Review.
-
Interferon gamma as an immune modulating adjunct therapy for invasive mucormycosis after severe burn - A case report.Front Immunol. 2022 Aug 22;13:883638. doi: 10.3389/fimmu.2022.883638. eCollection 2022. Front Immunol. 2022. PMID: 36072605 Free PMC article.
-
BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis.Sci Transl Med. 2020 May 6;12(542):eaax4517. doi: 10.1126/scitranslmed.aax4517. Sci Transl Med. 2020. PMID: 32376769 Free PMC article.
-
Ouabain Attenuates Sepsis-Induced Immunosuppression in Mice by Activation and Anti-Apoptosis of T Cells.Med Sci Monit. 2018 May 2;24:2720-2727. doi: 10.12659/MSM.906889. Med Sci Monit. 2018. PMID: 29717720 Free PMC article.
-
CD1d- and MR1-Restricted T Cells in Sepsis.Front Immunol. 2015 Aug 12;6:401. doi: 10.3389/fimmu.2015.00401. eCollection 2015. Front Immunol. 2015. PMID: 26322041 Free PMC article. Review.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Monneret G. How to identify systemic sepsis-induced immunoparalysis. Advances in Sepsis. 2005;4:42–49.
-
- Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. - PubMed
-
- Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM044118/GM/NIGMS NIH HHS/United States
- R01 GM072760/GM/NIGMS NIH HHS/United States
- F32 EY006765/EY/NEI NIH HHS/United States
- R01 GM055194-14/GM/NIGMS NIH HHS/United States
- R01 EY015570/EY/NEI NIH HHS/United States
- GM44118/GM/NIGMS NIH HHS/United States
- R37 GM044118-20/GM/NIGMS NIH HHS/United States
- R01 EY006765/EY/NEI NIH HHS/United States
- EY015570/EY/NEI NIH HHS/United States
- EY06765/EY/NEI NIH HHS/United States
- R01 AI057753/AI/NIAID NIH HHS/United States
- GM72760/GM/NIGMS NIH HHS/United States
- R01 GM055194/GM/NIGMS NIH HHS/United States
- GM55194/GM/NIGMS NIH HHS/United States
- R56 AI057753/AI/NIAID NIH HHS/United States
- R37 GM044118/GM/NIGMS NIH HHS/United States
- AI057753/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

