HAX-1 overexpression, splicing and cellular localization in tumors
- PMID: 20196840
- PMCID: PMC2843675
- DOI: 10.1186/1471-2407-10-76
HAX-1 overexpression, splicing and cellular localization in tumors
Abstract
Background: HAX-1 has been described as a protein potentially involved in carcinogenesis and especially metastasis. Its involvement in regulation of apoptosis and cell migration along with some data indicating its overexpression in cancer cell lines and tumors suggests that HAX-1 may play a role in neoplastic transformation. Here we present the first systematic analysis of HAX-1 expression in several solid tumors.
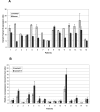
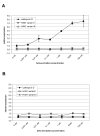
Methods: Using quantitative RT-PCR, we have determined the mRNA levels of HAX1 splice variant I in several solid tumors. We have also analyzed by semiquantitative and quantitative RT-PCR the expression of five HAX-1 splice variants in breast cancer samples and in normal tissue from the same individuals. Quantitative PCR was also employed to analyze the effect of estrogen on HAX1 expression in breast cancer cell line. Immunohistochemical analysis of HAX-1 was performed on normal and breast cancer samples.
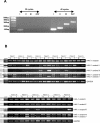
Results: The results reveal statistically important HAX1 up-regulation in breast cancer, lung cancer and melanoma, along with some minor variations in the splicing pattern. HAX-1 up-regulation in breast cancer samples was confirmed by immunohistochemical analysis, which also revealed an intriguing HAX-1 localization in the nuclei of the tumor cells, associated with strong ER status.
Conclusion: HAX-1 elevated levels in cancer tissues point to its involvement in neoplastic transformation, especially in breast cancer. The connection between HAX-1 nuclear location and ER status in breast cancer samples remains to be clarified.
Figures




Similar articles
-
HAX-1 promotes the migration and invasion of hepatocellular carcinoma cells through the induction of epithelial-mesenchymal transition via the NF-κB pathway.Exp Cell Res. 2019 Aug 1;381(1):66-76. doi: 10.1016/j.yexcr.2019.04.030. Epub 2019 Apr 30. Exp Cell Res. 2019. PMID: 31047882
-
Nuclear localization and cytosolic overexpression of LASP-1 correlates with tumor size and nodal-positivity of human breast carcinoma.BMC Cancer. 2007 Oct 23;7:198. doi: 10.1186/1471-2407-7-198. BMC Cancer. 2007. PMID: 17956604 Free PMC article.
-
Chemoresistance is associated with overexpression of HAX-1, inhibition of which resensitizes drug-resistant breast cancer cells to chemotherapy.Tumour Biol. 2017 Mar;39(3):1010428317692228. doi: 10.1177/1010428317692228. Tumour Biol. 2017. PMID: 28347249
-
HAX-1: a family of apoptotic regulators in health and disease.J Cell Physiol. 2011 Nov;226(11):2752-61. doi: 10.1002/jcp.22638. J Cell Physiol. 2011. PMID: 21302289 Review.
-
HAX-1: a multifunctional protein with emerging roles in human disease.Biochim Biophys Acta. 2009 Oct;1790(10):1139-48. doi: 10.1016/j.bbagen.2009.06.004. Epub 2009 Jun 12. Biochim Biophys Acta. 2009. PMID: 19524642 Review.
Cited by
-
HAX1 enhances the survival and metastasis of non-small cell lung cancer through the AKT/mTOR and MDM2/p53 signaling pathway.Thorac Cancer. 2020 Nov;11(11):3155-3167. doi: 10.1111/1759-7714.13634. Epub 2020 Sep 14. Thorac Cancer. 2020. PMID: 32926529 Free PMC article.
-
Hax-1 is rapidly degraded by the proteasome dependent on its PEST sequence.BMC Cell Biol. 2012 Jul 24;13:20. doi: 10.1186/1471-2121-13-20. BMC Cell Biol. 2012. PMID: 22827267 Free PMC article.
-
HAX1 impact on collective cell migration, cell adhesion, and cell shape is linked to the regulation of actomyosin contractility.Mol Biol Cell. 2019 Dec 1;30(25):3024-3036. doi: 10.1091/mbc.E19-05-0304. Epub 2019 Oct 23. Mol Biol Cell. 2019. PMID: 31644363 Free PMC article.
-
HAX-1 overexpression in gastric cancer promotes cell proliferation.Transl Cancer Res. 2020 Apr;9(4):2672-2682. doi: 10.21037/tcr.2020.02.69. Transl Cancer Res. 2020. PMID: 35117626 Free PMC article.
-
4R-Cembranoid Improves Outcomes after 6-Hydroxydopamine Challenge in Both In vitro and In vivo Models of Parkinson's Disease.Front Neurosci. 2017 May 29;11:272. doi: 10.3389/fnins.2017.00272. eCollection 2017. Front Neurosci. 2017. PMID: 28611572 Free PMC article.
References
-
- Sharp TV, Wang HW, Koumi A, Hollyman D, Endo Y, Ye H, Du MQ, Boshoff C. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. Journal of virology. 2002;76(2):802–816. doi: 10.1128/JVI.76.2.802-816.2002. - DOI - PMC - PubMed
-
- Matsuda G, Nakajima K, Kawaguchi Y, Yamanashi Y, Hirai K. Epstein-Barr virus (EBV) nuclear antigen leader protein (EBNA-LP) forms complexes with a cellular anti-apoptosis protein Bcl-2 or its EBV counterpart BHRF1 through HS1-associated protein X-1. Microbiology and immunology. 2003;47(1):91–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

