Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential
- PMID: 20182583
- PMCID: PMC2826823
Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential
Abstract
In eukaryotic cells, the endoplasmic reticulum (ER) is an organelle that is responsible for protein folding and assembly, lipid and sterol biosynthesis, and free calcium storage. In the past decade, intensive research effort has been focused on intracellular stress signaling pathways from the ER that lead to transcriptional and translational reprogramming of stressed cells. These signaling pathways, which are collectively termed Unfolded Protein Response (UPR), are critical for the cell to make survival or death decision under ER stress conditions. In recent years, research in the cancer field has revealed that ER stress and the UPR are highly induced in various tumors and are closely associated with cancer cell survival and resistance to anti-cancer treatments. Identifying the UPR components that are activated or suppressed in malignancy and exploring cancer therapeutic potentials by targeting the UPR are hot research spots. In this review, we summarize the recent progress in understating UPR signaling in cancer and its related therapeutic potential.
Keywords: ER stress; Endoplasmic reticulum; cancer; cancer therapy; malignancy; unfolded protein response.
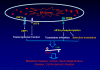
Figures
Similar articles
-
Proteostasis In The Endoplasmic Reticulum: Road to Cure.Cancers (Basel). 2019 Nov 14;11(11):1793. doi: 10.3390/cancers11111793. Cancers (Basel). 2019. PMID: 31739582 Free PMC article. Review.
-
Measurement of ER stress response and inflammation in the mouse model of nonalcoholic fatty liver disease.Methods Enzymol. 2011;489:329-48. doi: 10.1016/B978-0-12-385116-1.00019-4. Methods Enzymol. 2011. PMID: 21266239
-
Unfolded Protein Response Pathways in Neurodegenerative Diseases.J Mol Neurosci. 2015 Dec;57(4):529-37. doi: 10.1007/s12031-015-0633-3. Epub 2015 Aug 25. J Mol Neurosci. 2015. PMID: 26304853 Review.
-
Endoplasmic reticulum stress and unfolded protein response in inflammatory bowel disease.Inflamm Bowel Dis. 2015 Mar;21(3):636-44. doi: 10.1097/MIB.0000000000000238. Inflamm Bowel Dis. 2015. PMID: 25581827
-
Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease.Circ Res. 2010 Oct 29;107(9):1071-82. doi: 10.1161/CIRCRESAHA.110.227819. Circ Res. 2010. PMID: 21030724 Review.
Cited by
-
Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology.Signal Transduct Target Ther. 2022 Nov 19;7(1):379. doi: 10.1038/s41392-022-01243-0. Signal Transduct Target Ther. 2022. PMID: 36402753 Free PMC article. Review.
-
The endoplasmic reticulum stress response in aging and age-related diseases.Front Physiol. 2012 Jul 16;3:263. doi: 10.3389/fphys.2012.00263. eCollection 2012. Front Physiol. 2012. PMID: 22934019 Free PMC article.
-
Endoplasmic Reticulum Stress and miRNA Impairment in Aging and Age-Related Diseases.Front Aging. 2022 Jan 20;2:790702. doi: 10.3389/fragi.2021.790702. eCollection 2021. Front Aging. 2022. PMID: 35822008 Free PMC article. Review.
-
Unfolded Protein Response (UPR) in Survival, Dormancy, Immunosuppression, Metastasis, and Treatments of Cancer Cells.Int J Mol Sci. 2019 May 22;20(10):2518. doi: 10.3390/ijms20102518. Int J Mol Sci. 2019. PMID: 31121863 Free PMC article. Review.
-
The autophagy-related marker LC3 can predict prognosis in human hepatocellular carcinoma.PLoS One. 2013 Nov 25;8(11):e81540. doi: 10.1371/journal.pone.0081540. eCollection 2013. PLoS One. 2013. PMID: 24282606 Free PMC article.
References
-
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. - PubMed
-
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. - PubMed
-
- McMillian DR, Gething MJ, Sambrook J. The cellular response to unfolded proteins: Intercom-partmental Signaling. Current Opinions in Biotechnology. 1994;5:540–545. - PubMed
-
- Sidrauski C, Chapman R, Walter P. The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends. Cell Biol. 1998;8:245–249. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
