Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases
- PMID: 20181693
- PMCID: PMC2863753
- DOI: 10.1128/JVI.02406-09
Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases
Abstract
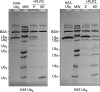
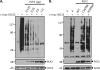
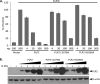
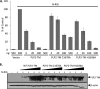
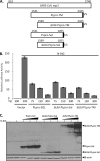
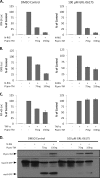
Coronaviruses encode multifunctional proteins that are critical for viral replication and for blocking the innate immune response to viral infection. One such multifunctional domain is the coronavirus papain-like protease (PLP), which processes the viral replicase polyprotein, has deubiquitinating (DUB) activity, and antagonizes the induction of type I interferon (IFN). Here we characterized the DUB and IFN antagonism activities of the PLP domains of human coronavirus NL63 and severe acute respiratory syndrome (SARS) coronavirus to determine if DUB activity mediates interferon antagonism. We found that NL63 PLP2 deconjugated ubiquitin (Ub) and the Ub-line molecule ISG15 from cellular substrates and processed both lysine-48- and lysine-63- linked polyubiquitin chains. This PLP2 DUB activity was dependent on an intact catalytic cysteine residue. We demonstrated that in contrast to PLP2 DUB activity, PLP2-mediated interferon antagonism did not require enzymatic activity. Furthermore, addition of an inhibitor that blocks coronavirus protease/DUB activity did not abrogate interferon antagonism. These results indicated that a component of coronavirus PLP-mediated interferon antagonism was independent of protease and DUB activity. Overall, these results demonstrate the multifunctional nature of the coronavirus PLP domain as a viral protease, DUB, and IFN antagonist and suggest that these independent activities may provide multiple targets for antiviral therapies.
Figures


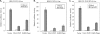
Similar articles
-
Structure-Guided Mutagenesis Alters Deubiquitinating Activity and Attenuates Pathogenesis of a Murine Coronavirus.J Virol. 2020 May 18;94(11):e01734-19. doi: 10.1128/JVI.01734-19. Print 2020 May 18. J Virol. 2020. PMID: 32188728 Free PMC article.
-
Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating activity of SARS-CoV papain-like protease.PLoS Pathog. 2014 May 22;10(5):e1004113. doi: 10.1371/journal.ppat.1004113. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24854014 Free PMC article.
-
Proteolytic processing and deubiquitinating activity of papain-like proteases of human coronavirus NL63.J Virol. 2007 Jun;81(11):6007-18. doi: 10.1128/JVI.02747-06. Epub 2007 Mar 28. J Virol. 2007. PMID: 17392370 Free PMC article.
-
The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds.Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub 2014 Dec 29. Antiviral Res. 2015. PMID: 25554382 Free PMC article. Review.
-
Nidovirus papain-like proteases: multifunctional enzymes with protease, deubiquitinating and deISGylating activities.Virus Res. 2014 Dec 19;194:184-90. doi: 10.1016/j.virusres.2014.01.025. Epub 2014 Feb 7. Virus Res. 2014. PMID: 24512893 Free PMC article. Review.
Cited by
-
Role of Virally-Encoded Deubiquitinating Enzymes in Regulation of the Virus Life Cycle.Int J Mol Sci. 2021 Apr 23;22(9):4438. doi: 10.3390/ijms22094438. Int J Mol Sci. 2021. PMID: 33922750 Free PMC article. Review.
-
ISGylation of the SARS-CoV-2 N protein by HERC5 impedes N oligomerization and thereby viral RNA synthesis.J Virol. 2024 Sep 17;98(9):e0086924. doi: 10.1128/jvi.00869-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194248
-
Research Advances on Swine Acute Diarrhea Syndrome Coronavirus.Animals (Basel). 2024 Jan 30;14(3):448. doi: 10.3390/ani14030448. Animals (Basel). 2024. PMID: 38338091 Free PMC article. Review.
-
Viral evasion of intracellular DNA and RNA sensing.Nat Rev Microbiol. 2016 Jun;14(6):360-73. doi: 10.1038/nrmicro.2016.45. Epub 2016 May 13. Nat Rev Microbiol. 2016. PMID: 27174148 Free PMC article. Review.
-
From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses.Infect Genet Evol. 2020 Nov;85:104502. doi: 10.1016/j.meegid.2020.104502. Epub 2020 Aug 13. Infect Genet Evol. 2020. PMID: 32798769 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

