Presenilins and the gamma-secretase: still a complex problem
- PMID: 20181106
- PMCID: PMC2845129
- DOI: 10.1186/1756-6606-3-7
Presenilins and the gamma-secretase: still a complex problem
Abstract
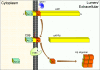
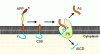
The presenilins form part of a complex of membrane proteins that are involved in the proteolytic cleavage of cell-surface molecules. This article reviews the history of the discovery of the presenilins, their role in the pathogenesis of Alzheimer's disease and in the metabolism of the amyloid-beta precursor protein. Unanswered questions about their biochemical mechanism of action and their effects on Ca2+ homeostasis are examined.
Figures
Similar articles
-
Presenilins: how much more than γ-secretase?!Biochem Soc Trans. 2010 Dec;38(6):1474-8. doi: 10.1042/BST0381474. Biochem Soc Trans. 2010. PMID: 21118110
-
Presenilins as endoplasmic reticulum calcium leak channels and Alzheimer's disease pathogenesis.Sci China Life Sci. 2011 Aug;54(8):744-51. doi: 10.1007/s11427-011-4201-y. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786197 Review.
-
Beyond γ-secretase activity: The multifunctional nature of presenilins in cell signalling pathways.Cell Signal. 2016 Jan;28(1):1-11. doi: 10.1016/j.cellsig.2015.10.006. Epub 2015 Oct 21. Cell Signal. 2016. PMID: 26498858 Review.
-
Nicastrin is dispensable for gamma-secretase protease activity in the presence of specific presenilin mutations.J Biol Chem. 2009 May 8;284(19):13013-22. doi: 10.1074/jbc.M807653200. Epub 2009 Mar 2. J Biol Chem. 2009. PMID: 19254953 Free PMC article.
-
Mutations in amyloid precursor protein affect its interactions with presenilin/gamma-secretase.Mol Cell Neurosci. 2009 Jun;41(2):166-74. doi: 10.1016/j.mcn.2009.02.008. Epub 2009 Mar 9. Mol Cell Neurosci. 2009. PMID: 19281847 Free PMC article.
Cited by
-
Role of gamma-secretase in human umbilical-cord derived mesenchymal stem cell mediated suppression of NK cell cytotoxicity.Cell Commun Signal. 2014 Sep 30;12:63. doi: 10.1186/s12964-014-0063-9. Cell Commun Signal. 2014. PMID: 25266361 Free PMC article.
-
Gene × environment interaction by a longitudinal epigenome-wide association study (LEWAS) overcomes limitations of genome-wide association study (GWAS).Epigenomics. 2012 Dec;4(6):685-99. doi: 10.2217/epi.12.60. Epigenomics. 2012. PMID: 23244313 Free PMC article.
-
Discovery of candidate genes for muscle traits based on GWAS supported by eQTL-analysis.Int J Biol Sci. 2014 Mar 10;10(3):327-37. doi: 10.7150/ijbs.8134. eCollection 2014. Int J Biol Sci. 2014. PMID: 24643240 Free PMC article.
-
Huntingtin cleavage product A forms in neurons and is reduced by gamma-secretase inhibitors.Mol Neurodegener. 2010 Dec 14;5:58. doi: 10.1186/1750-1326-5-58. Mol Neurodegener. 2010. PMID: 21156064 Free PMC article.
-
HPRT deficiency coordinately dysregulates canonical Wnt and presenilin-1 signaling: a neuro-developmental regulatory role for a housekeeping gene?PLoS One. 2011 Jan 28;6(1):e16572. doi: 10.1371/journal.pone.0016572. PLoS One. 2011. PMID: 21305049 Free PMC article.
References
-
- Storey E, Kinsella GJ, Slavin MJ. The neuropsychological diagnosis of Alzheimer's disease. J Alzheimers Dis. 2001;3:261–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

