Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet
- PMID: 20176722
- PMCID: PMC2850229
- DOI: 10.1210/en.2009-1019
Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet
Abstract
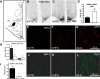
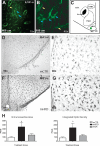
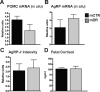
The hypothalamic melanocortin system, which controls appetite and energy expenditure, develops during the third trimester in primates. Thus, maternal nutrition and health may have a profound influence on the development of this system. To study the effects of chronic maternal high-fat diet (HFD) on the development of the melanocortin system in the fetal nonhuman primate, we placed adult female macaques on either a control (CTR) diet or a HFD for up to 4 yr. A subgroup of adult female HFD animals was also switched to CTR diet during the fifth year of the study (diet reversal). Third-trimester fetuses from mothers on HFD showed increases in proopiomelanocortin mRNA expression, whereas agouti-related protein mRNA and peptide levels were decreased in comparison with CTR fetuses. Proinflammatory cytokines, including IL-1beta and IL-1 type 1 receptor, and markers of activated microglia were elevated in the hypothalamus, suggesting an activation of the local inflammatory response. Fetuses of diet-reversal mothers had normal melanocortin levels. These results raise the concern that chronic consumption of a HFD during pregnancy, independent of maternal obesity and diabetes, can lead to widespread activation of proinflammatory cytokines that may alter the development of the melanocortin system. The abnormalities in the fetal POMC system, if maintained into the postnatal period, could impact several systems, including body weight homeostasis, stress responses, and cardiovascular function. Indeed, the HFD offspring develop early-onset excess weight gain. These abnormalities may be prevented by healthful nutrient consumption during pregnancy even in obese and severely insulin-resistant individuals.
Figures
Similar articles
-
Maternal and postnatal high-fat diet consumption programs energy balance and hypothalamic melanocortin signaling in nonhuman primate offspring.Am J Physiol Regul Integr Comp Physiol. 2017 Aug 1;313(2):R169-R179. doi: 10.1152/ajpregu.00309.2016. Epub 2017 Apr 12. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28404581 Free PMC article.
-
Maternal high-fat diet impacts endothelial function in nonhuman primate offspring.Int J Obes (Lond). 2013 Feb;37(2):254-62. doi: 10.1038/ijo.2012.42. Epub 2012 Mar 27. Int J Obes (Lond). 2013. PMID: 22450853 Free PMC article.
-
Respective contributions of maternal insulin resistance and diet to metabolic and hypothalamic phenotypes of progeny.Obesity (Silver Spring). 2011 Mar;19(3):492-9. doi: 10.1038/oby.2010.245. Epub 2010 Oct 14. Obesity (Silver Spring). 2011. PMID: 20948526 Free PMC article.
-
Perinatal nutrition programs the hypothalamic melanocortin system in offspring.Horm Metab Res. 2013 Dec;45(13):980-90. doi: 10.1055/s-0033-1357182. Epub 2013 Oct 24. Horm Metab Res. 2013. PMID: 24158879 Review.
-
Maternal high fat diets: impacts on offspring obesity and epigenetic hypothalamic programming.Front Genet. 2023 May 11;14:1158089. doi: 10.3389/fgene.2023.1158089. eCollection 2023. Front Genet. 2023. PMID: 37252665 Free PMC article. Review.
Cited by
-
Consumption of Substances of Abuse during Pregnancy Increases Consumption in Offspring: Possible Underlying Mechanisms.Front Nutr. 2016 Apr 20;3:11. doi: 10.3389/fnut.2016.00011. eCollection 2016. Front Nutr. 2016. PMID: 27148536 Free PMC article. Review.
-
Neurodevelopment Is Dependent on Maternal Diet: Placenta and Brain Glucose Transporters GLUT1 and GLUT3.Nutrients. 2024 Jul 21;16(14):2363. doi: 10.3390/nu16142363. Nutrients. 2024. PMID: 39064806 Free PMC article. Review.
-
Effects of diabetes on microglial physiology: a systematic review of in vitro, preclinical and clinical studies.J Neuroinflammation. 2023 Mar 3;20(1):57. doi: 10.1186/s12974-023-02740-x. J Neuroinflammation. 2023. PMID: 36869375 Free PMC article. Review.
-
N-Acetylcysteine Resolves Placental Inflammatory-Vasculopathic Changes in Mice Consuming a High-Fat Diet.Am J Pathol. 2019 Nov;189(11):2246-2257. doi: 10.1016/j.ajpath.2019.07.010. Epub 2019 Aug 17. Am J Pathol. 2019. PMID: 31430466 Free PMC article.
-
Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition.Endocrinology. 2011 Jun;152(6):2456-64. doi: 10.1210/en.2010-1332. Epub 2011 Mar 29. Endocrinology. 2011. PMID: 21447636 Free PMC article.
References
-
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS 1997 Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 - PubMed
-
- Cone RD 2005 Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 - PubMed
-
- Farooqi IS, O'Rahilly S 2008 Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 4:569–577 - PubMed
-
- Farooqi IS, O'Rahilly S 2007 Genetic factors in human obesity. Obes Rev 8(Suppl 1):37–40 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

