Antineoplastic effects of an Aurora B kinase inhibitor in breast cancer
- PMID: 20175926
- PMCID: PMC2839967
- DOI: 10.1186/1476-4598-9-42
Antineoplastic effects of an Aurora B kinase inhibitor in breast cancer
Abstract
Background: Aurora B kinase is an important mitotic kinase involved in chromosome segregation and cytokinesis. It is overexpressed in many cancers and thus may be an important molecular target for chemotherapy. AZD1152 is the prodrug for AZD1152-HQPA, which is a selective inhibitor of Aurora B kinase activity. Preclinical antineoplastic activity of AZD1152 against acute myelogenous leukemia, multiple myeloma and colorectal cancer has been reported. However, this compound has not been evaluated in breast cancer, the second leading cause of cancer deaths among women.
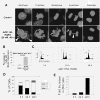
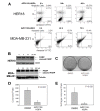
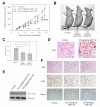
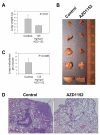
Results: The antineoplastic activity of AZD1152-HQPA in six human breast cancer cell lines, three of which overexpress HER2, is demonstrated. AZD1152-HQPA specifically inhibited Aurora B kinase activity in breast cancer cells, thereby causing mitotic catastrophe, polyploidy and apoptosis, which in turn led to apoptotic death. AZD1152 administration efficiently suppressed the tumor growth in a breast cancer cell xenograft model. In addition, AZD1152 also inhibited pulmonary metastatic nodule formation in a metastatic breast cancer model. Notably, it was also found that the protein level of Aurora B kinase declined after inhibition of Aurora B kinase activity by AZD1152-HQPA in a time- and dose-dependent manner. Investigation of the underlying mechanism suggested that AZD1152-HQPA accelerated protein turnover of Aurora B via enhancing its ubiquitination.
Conclusions: It was shown that AZD1152 is an effective antineoplastic agent for breast cancer, and our results define a novel mechanism for posttranscriptional regulation of Aurora B after AZD1152 treatment and provide insight into dosing regimen design for this kinase inhibitor in metastatic breast cancer treatment.
Figures

Similar articles
-
Inhibitor of Aurora Kinase B Induces Differentially Cell Death and Polyploidy via DNA Damage Response Pathways in Neurological Malignancy: Shedding New Light on the Challenge of Resistance to AZD1152-HQPA.Mol Neurobiol. 2016 Apr;53(3):1808-1823. doi: 10.1007/s12035-015-9139-9. Epub 2015 Mar 11. Mol Neurobiol. 2016. PMID: 25752998
-
Aurora B kinase inhibitor AZD1152: determinants of action and ability to enhance chemotherapeutics effectiveness in pancreatic and colon cancer.Br J Cancer. 2011 Mar 1;104(5):769-80. doi: 10.1038/bjc.2011.21. Epub 2011 Feb 8. Br J Cancer. 2011. PMID: 21304529 Free PMC article.
-
The FLT3 internal tandem duplication mutation is a secondary target of the aurora B kinase inhibitor AZD1152-HQPA in acute myelogenous leukemia cells.Mol Cancer Ther. 2010 Mar;9(3):661-72. doi: 10.1158/1535-7163.MCT-09-1144. Epub 2010 Feb 16. Mol Cancer Ther. 2010. PMID: 20159992
-
Aurora-B kinase inhibitors for cancer chemotherapy.Mini Rev Med Chem. 2008 Dec;8(14):1514-25. doi: 10.2174/138955708786786480. Mini Rev Med Chem. 2008. PMID: 19075809 Review.
-
Aurora kinase inhibitors as anti-cancer therapy.Anticancer Drugs. 2010 Apr;21(4):339-50. doi: 10.1097/CAD.0b013e3283350dd1. Anticancer Drugs. 2010. PMID: 20016367 Review.
Cited by
-
Derivation of stationary distributions of biochemical reaction networks via structure transformation.Commun Biol. 2021 May 24;4(1):620. doi: 10.1038/s42003-021-02117-x. Commun Biol. 2021. PMID: 34031517 Free PMC article.
-
The transcription factor YY1 is a novel substrate for Aurora B kinase at G2/M transition of the cell cycle.PLoS One. 2012;7(11):e50645. doi: 10.1371/journal.pone.0050645. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226345 Free PMC article.
-
HSET overexpression fuels tumor progression via centrosome clustering-independent mechanisms in breast cancer patients.Oncotarget. 2015 Mar 20;6(8):6076-91. doi: 10.18632/oncotarget.3475. Oncotarget. 2015. PMID: 25788277 Free PMC article.
-
Inhibition of Cdc20 suppresses the metastasis in triple negative breast cancer (TNBC).Breast Cancer. 2021 Sep;28(5):1073-1086. doi: 10.1007/s12282-021-01242-z. Epub 2021 Apr 3. Breast Cancer. 2021. PMID: 33813687
-
SU6668 suppresses proliferation of triple negative breast cancer cells through down-regulating MTDH expression.Cancer Cell Int. 2013 Aug 29;13(1):88. doi: 10.1186/1475-2867-13-88. Cancer Cell Int. 2013. PMID: 23984913 Free PMC article.
References
-
- Yang J, Ikezoe T, Nishioka C, Tasaka T, Taniguchi A, Kuwayama Y, Komatsu N, Bandobashi K, Togitani K, Koeffler HP. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110:2034–2040. doi: 10.1182/blood-2007-02-073700. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

