SAT1, A Glutamine Transporter, is Preferentially Expressed in GABAergic Neurons
- PMID: 20161990
- PMCID: PMC2820376
- DOI: 10.3389/neuro.05.001.2010
SAT1, A Glutamine Transporter, is Preferentially Expressed in GABAergic Neurons
Abstract
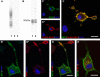
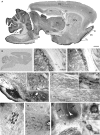
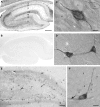
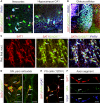
Subsets of GABAergic neurons are able to maintain high frequency discharge patterns, which requires efficient replenishment of the releasable pool of GABA. Although glutamine is considered a preferred precursor of GABA, the identity of transporters involved in glutamine uptake by GABAergic neurons remains elusive. Molecular analyses revealed that SAT1 (Slc38a1) features system A characteristics with a preferential affinity for glutamine, and that SAT1 mRNA expression is associated with GABAergic neurons. By generating specific antibodies against SAT1 we show that this glutamine carrier is particularly enriched in GABAergic neurons. Cellular SAT1 distribution resembles that of GAD67, an essential GABA synthesis enzyme, suggesting that SAT1 can be involved in translocating glutamine into GABAergic neurons to facilitate inhibitory neurotransmitter generation.
Keywords: GAD67; SNAT1; Slc38a1; glutamate/GABA-glutamine cycle; interneuron; neurotransmitter; parvalbumin; system A.
Figures


Similar articles
-
Slc38a1 Conveys Astroglia-Derived Glutamine into GABAergic Interneurons for Neurotransmitter GABA Synthesis.Cells. 2020 Jul 13;9(7):1686. doi: 10.3390/cells9071686. Cells. 2020. PMID: 32668809 Free PMC article.
-
The Glutamine Transporter Slc38a1 Regulates GABAergic Neurotransmission and Synaptic Plasticity.Cereb Cortex. 2019 Dec 17;29(12):5166-5179. doi: 10.1093/cercor/bhz055. Cereb Cortex. 2019. PMID: 31050701 Free PMC article.
-
Functional properties and cellular distribution of the system A glutamine transporter SNAT1 support specialized roles in central neurons.J Biol Chem. 2003 Jun 27;278(26):23720-30. doi: 10.1074/jbc.M212718200. Epub 2003 Apr 8. J Biol Chem. 2003. PMID: 12684517
-
Chronic hyperammonemia alters extracellular glutamate, glutamine and GABA and membrane expression of their transporters in rat cerebellum. Modulation by extracellular cGMP.Neuropharmacology. 2019 Dec 15;161:107496. doi: 10.1016/j.neuropharm.2019.01.011. Epub 2019 Jan 11. Neuropharmacology. 2019. PMID: 30641078 Review.
-
The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism.Neurochem Res. 2015 Feb;40(2):402-9. doi: 10.1007/s11064-014-1473-1. Epub 2014 Nov 8. Neurochem Res. 2015. PMID: 25380696 Review.
Cited by
-
Protein Kinase C Phosphorylates the System N Glutamine Transporter SN1 (Slc38a3) and Regulates Its Membrane Trafficking and Degradation.Front Endocrinol (Lausanne). 2013 Oct 2;4:138. doi: 10.3389/fendo.2013.00138. Front Endocrinol (Lausanne). 2013. PMID: 24106489 Free PMC article. Review.
-
Glutamine supplementation in a child with inherited GS deficiency improves the clinical status and partially corrects the peripheral and central amino acid imbalance.Orphanet J Rare Dis. 2012 Jul 25;7:48. doi: 10.1186/1750-1172-7-48. Orphanet J Rare Dis. 2012. PMID: 22830360 Free PMC article.
-
The Transcriptomic Analysis of NSC-34 Motor Neuron-Like Cells Reveals That Cannabigerol Influences Synaptic Pathways: A Comparative Study with Cannabidiol.Life (Basel). 2020 Oct 1;10(10):227. doi: 10.3390/life10100227. Life (Basel). 2020. PMID: 33019509 Free PMC article.
-
Inhibition of the glutamine transporter SNAT1 confers neuroprotection in mice by modulating the mTOR-autophagy system.Commun Biol. 2019 Sep 18;2:346. doi: 10.1038/s42003-019-0582-4. eCollection 2019. Commun Biol. 2019. PMID: 31552299 Free PMC article.
-
Physiological Expression of Ion Channel Receptors in Human Periodontal Ligament Stem Cells.Cells. 2019 Mar 6;8(3):219. doi: 10.3390/cells8030219. Cells. 2019. PMID: 30845727 Free PMC article.
References
-
- Armano S., Coco S., Bacci A., Pravettoni E., Schenk U., Verderio C., Varoqui H., Erickson J. D., Matteoli M. (2002). Localization and functional relevance of system a neutral amino acid transporters in cultured hippocampal neurons. J. Biol. Chem. 277, 10467–1047310.1074/jbc.M110942200 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

