CXCR7 functions as a scavenger for CXCL12 and CXCL11
- PMID: 20161793
- PMCID: PMC2820091
- DOI: 10.1371/journal.pone.0009175
CXCR7 functions as a scavenger for CXCL12 and CXCL11
Abstract
Background: CXCR7 (RDC1), the recently discovered second receptor for CXCL12, is phylogenetically closely related to chemokine receptors, but fails to couple to G-proteins and to induce typical chemokine receptor mediated cellular responses. The function of CXCR7 is controversial. Some studies suggest a signaling activity in mammalian cells and zebrafish embryos, while others indicate a decoy activity in fish. Here we investigated the two propositions in human tissues.
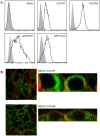
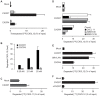
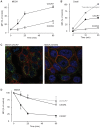
Methodology/principal findings: We provide evidence and mechanistic insight that CXCR7 acts as specific scavenger for CXCL12 and CXCL11 mediating effective ligand internalization and targeting of the chemokine cargo for degradation. Consistently, CXCR7 continuously cycles between the plasma membrane and intracellular compartments in the absence and presence of ligand, both in mammalian cells and in zebrafish. In accordance with the proposed activity as a scavenger receptor CXCR7-dependent chemokine degradation does not become saturated with increasing ligand concentrations. Active CXCL12 sequestration by CXCR7 is demonstrated in adult mouse heart valves and human umbilical vein endothelium.
Conclusions/significance: The finding that CXCR7 specifically scavenges CXCL12 suggests a critical function of the receptor in modulating the activity of the ubiquitously expressed CXCR4 in development and tumor formation. Scavenger activity of CXCR7 might also be important for the fine tuning of the mobility of hematopoietic cells in the bone marrow and lymphoid organs.
Conflict of interest statement
Figures
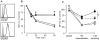

Similar articles
-
Imaging ligand-dependent activation of CXCR7.Neoplasia. 2009 Oct;11(10):1022-35. doi: 10.1593/neo.09724. Neoplasia. 2009. PMID: 19794961 Free PMC article.
-
Constitutive and chemokine-dependent internalization and recycling of CXCR7 in breast cancer cells to degrade chemokine ligands.Oncogene. 2010 Aug 12;29(32):4599-610. doi: 10.1038/onc.2010.212. Epub 2010 Jun 7. Oncogene. 2010. PMID: 20531309 Free PMC article.
-
Ligand-specific conformational transitions and intracellular transport are required for atypical chemokine receptor 3-mediated chemokine scavenging.J Biol Chem. 2018 Jan 19;293(3):893-905. doi: 10.1074/jbc.M117.814947. Epub 2017 Nov 27. J Biol Chem. 2018. PMID: 29180449 Free PMC article.
-
CXCR7 impact on CXCL12 biology and disease.Trends Mol Med. 2013 Jan;19(1):12-22. doi: 10.1016/j.molmed.2012.10.004. Epub 2012 Nov 12. Trends Mol Med. 2013. PMID: 23153575 Review.
-
Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12.Cytokine Growth Factor Rev. 2013 Feb;24(1):41-9. doi: 10.1016/j.cytogfr.2012.08.007. Epub 2012 Sep 16. Cytokine Growth Factor Rev. 2013. PMID: 22989616 Free PMC article. Review.
Cited by
-
The chemokine CXCL12 and the HIV-1 envelope protein gp120 regulate spontaneous activity of Cajal-Retzius cells in opposite directions.J Physiol. 2012 Jul 1;590(13):3185-202. doi: 10.1113/jphysiol.2011.224873. Epub 2012 Apr 2. J Physiol. 2012. PMID: 22473778 Free PMC article.
-
Discovery of Diphenylacetamides as CXCR7 Inhibitors with Novel β-Arrestin Antagonist Activity.ACS Med Chem Lett. 2020 May 14;11(6):1330-1334. doi: 10.1021/acsmedchemlett.0c00163. eCollection 2020 Jun 11. ACS Med Chem Lett. 2020. PMID: 32551020 Free PMC article.
-
Microfluidic investigation of BDNF-enhanced neural stem cell chemotaxis in CXCL12 gradients.Small. 2013 Feb 25;9(4):585-95. doi: 10.1002/smll.201202208. Epub 2012 Oct 26. Small. 2013. PMID: 23109183 Free PMC article.
-
A new obligate CXCL4-CXCL12 heterodimer for studying chemokine heterodimer activities and mechanisms.Sci Rep. 2022 Oct 13;12(1):17204. doi: 10.1038/s41598-022-21651-0. Sci Rep. 2022. PMID: 36229490 Free PMC article.
-
Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells.Oncogene. 2012 Nov 8;31(45):4750-8. doi: 10.1038/onc.2011.633. Epub 2012 Jan 23. Oncogene. 2012. PMID: 22266857 Free PMC article.
References
-
- Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9:953–959. - PubMed
-
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. - PubMed
-
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. - PubMed
-
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

