CHARMM Additive All-Atom Force Field for Acyclic Polyalcohols, Acyclic Carbohydrates and Inositol
- PMID: 20160980
- PMCID: PMC2760998
- DOI: 10.1021/ct9000608
CHARMM Additive All-Atom Force Field for Acyclic Polyalcohols, Acyclic Carbohydrates and Inositol
Abstract
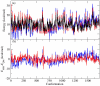
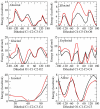
Parametrization of the additive all-atom CHARMM force field for acyclic polyalcohols, acyclic carbohydrates and inositol is conducted. Initial parameters were transferred from the alkanes and hexopyranose carbohydrates, with subsequent development and optimization of parameters unique to the molecules considered in this study. Using the model compounds acetone and acetaldehyde, nonbonded parameters for carbonyls were optimized targeting quantum mechanical interaction data for solute-water pairs and pure solvent thermodynamic data. Bond and angle parameters were adjusted by comparing optimized geometries to small molecule crystal survey data and by performing vibrational analyses on acetone, acetaldehyde and glycerol. C-C-C-C, C-C-C-O, C-C-OH and O-C-C-O torsional parameters for polyol chains were fit to quantum mechanical dihedral potential energy scans comprising over 1500 RIMP2/cc-pVTZ//MP2/6-31G(d) conformations using an automated Monte Carlo simulated annealing procedure. Comparison of computed condensed-phase data, including crystal lattice parameters and densities, NMR proton-proton couplings, densities and diffusion coefficients of aqueous solutions, to experimental data validated the optimized parameters. Parameter development for these compounds proved particularly challenging because of the flexibility of the acyclic sugars and polyalcohols as well as the intramolecular hydrogen bonding between vicinal hydroxyls for all of the compounds. The newly optimized additive CHARMM force field parameters are anticipated to be of utility for atomic level of detail simulations of acyclic polyalcohols, acyclic carbohydrates and inositol in solution.
Figures

Similar articles
-
CHARMM Additive All-Atom Force Field for Glycosidic Linkages between Hexopyranoses.J Chem Theory Comput. 2009 Aug 20;5(9):2353-2370. doi: 10.1021/ct900242e. J Chem Theory Comput. 2009. PMID: 20161005 Free PMC article.
-
CHARMM additive all-atom force field for glycosidic linkages in carbohydrates involving furanoses.J Phys Chem B. 2010 Oct 14;114(40):12981-94. doi: 10.1021/jp105758h. J Phys Chem B. 2010. PMID: 20845956 Free PMC article.
-
CHARMM additive all-atom force field for aldopentofuranoses, methyl-aldopentofuranosides, and fructofuranose.J Phys Chem B. 2009 Sep 17;113(37):12466-76. doi: 10.1021/jp905496e. J Phys Chem B. 2009. PMID: 19694450 Free PMC article.
-
CHARMM Additive All-Atom Force Field for Phosphate and Sulfate Linked to Carbohydrates.J Chem Theory Comput. 2012 Feb 14;8(2):759-776. doi: 10.1021/ct200792v. Epub 2011 Dec 26. J Chem Theory Comput. 2012. PMID: 22685386 Free PMC article.
-
Biomolecular force fields: where have we been, where are we now, where do we need to go and how do we get there?J Comput Aided Mol Des. 2019 Feb;33(2):133-203. doi: 10.1007/s10822-018-0111-4. Epub 2018 Nov 30. J Comput Aided Mol Des. 2019. PMID: 30506158 Review.
Cited by
-
Conformational determinants of the activity of antiproliferative factor glycopeptide.J Chem Inf Model. 2013 May 24;53(5):1127-37. doi: 10.1021/ci400147s. Epub 2013 May 15. J Chem Inf Model. 2013. PMID: 23627670 Free PMC article.
-
CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling.J Chem Theory Comput. 2011 Oct 11;7(10):3162-3180. doi: 10.1021/ct200328p. J Chem Theory Comput. 2011. PMID: 22125473 Free PMC article.
-
CHARMM Drude Polarizable Force Field for Glycosidic Linkages Involving Pyranoses and Furanoses.J Chem Theory Comput. 2018 Jun 12;14(6):3132-3143. doi: 10.1021/acs.jctc.8b00175. Epub 2018 May 4. J Chem Theory Comput. 2018. PMID: 29694037 Free PMC article.
-
CHARMM additive and polarizable force fields for biophysics and computer-aided drug design.Biochim Biophys Acta. 2015 May;1850(5):861-871. doi: 10.1016/j.bbagen.2014.08.004. Epub 2014 Aug 19. Biochim Biophys Acta. 2015. PMID: 25149274 Free PMC article. Review.
-
Molecular mechanics.Curr Pharm Des. 2014;20(20):3281-92. doi: 10.2174/13816128113199990600. Curr Pharm Des. 2014. PMID: 23947650 Free PMC article. Review.
References
-
- Costantino L, Rastelli G, Vescovini K, Cignarella G, Vianello P, DelCorso A, Cappiello M, Mura U, Barlocco D. J. Med. Chem. 1996;39:4396. - PubMed
-
- Singh SB, Malamas MS, Hohman TC, Nilakantan R, Carper DA, Kitchen D. J. Med. Chem. 2000;43:1062. - PubMed
-
- de la Fuente JA, Manzanaro S, Martin MJ, de Quesada TG, Reymundo I, Luengo SM, Gago F. J. Med. Chem. 2003;46:5208. - PubMed
-
- Berridge MJ, Irvine RF. Nature. 1984;312:315. - PubMed
-
- Volpe P, Salviati G, Di Virgilio F, Pozzan T. Nature. 1985;316:347. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
