Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis
- PMID: 20160348
- PMCID: PMC2827960
- DOI: 10.1172/JCI41013
Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis
Abstract
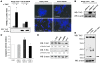
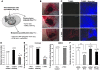
Tropomyosin-related kinase receptor C (TrkC) is a neurotrophin receptor with tyrosine kinase activity that was expected to be oncogenic. However, it has several characteristics of a tumor suppressor: its expression in tumors has often been associated with good prognosis; and it was recently demonstrated to be a dependence receptor, transducing different positive signals in the presence of ligand but inducing apoptosis in the absence of ligand. Here we show that the TrkC ligand neurotrophin-3 (NT-3) is upregulated in a large fraction of aggressive human neuroblastomas (NBs) and that it blocks TrkC-induced apoptosis of human NB cell lines, consistent with the idea that TrkC is a dependence receptor. Functionally, both siRNA knockdown of NT-3 expression and incubation with a TrkC-specific blocking antibody triggered apoptosis in human NB cell lines. Importantly, disruption of the NT-3 autocrine loop in malignant human neuroblasts triggered in vitro NB cell death and inhibited tumor growth and metastasis in both a chick and a mouse xenograft model. Thus, we believe that our data suggest that NT-3/TrkC disruption is a putative alternative targeted therapeutic strategy for the treatment of NB.
Figures


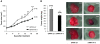
Similar articles
-
Targeting neurotrophin-3 and its dependence receptor tyrosine kinase receptor C: a new antitumoral strategy.Expert Opin Ther Targets. 2011 Jul;15(7):847-58. doi: 10.1517/14728222.2011.575361. Epub 2011 Apr 8. Expert Opin Ther Targets. 2011. PMID: 21473736 Review.
-
The Neurotrophin Receptor TrkC as a Novel Molecular Target of the Antineuroblastoma Action of Valproic Acid.Int J Mol Sci. 2021 Jul 21;22(15):7790. doi: 10.3390/ijms22157790. Int J Mol Sci. 2021. PMID: 34360553 Free PMC article.
-
Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival.Clin Cancer Res. 2011 Apr 1;17(7):1741-52. doi: 10.1158/1078-0432.CCR-10-1890. Epub 2011 Feb 24. Clin Cancer Res. 2011. PMID: 21350004
-
Differential Clinical Significance of Neurotrophin-3 Expression according to MYCN Amplification and TrkC Expression in Neuroblastoma.J Korean Med Sci. 2019 Oct 14;34(39):e254. doi: 10.3346/jkms.2019.34.e254. J Korean Med Sci. 2019. PMID: 31602824 Free PMC article.
-
Neurotrophin-3 in the development of the enteric nervous system.Prog Brain Res. 2004;146:243-63. doi: 10.1016/S0079-6123(03)46016-0. Prog Brain Res. 2004. PMID: 14699968 Review.
Cited by
-
Delayed peripheral treatment with neurotrophin-3 improves sensorimotor recovery after central nervous system injury.Neural Regen Res. 2019 Oct;14(10):1703-1704. doi: 10.4103/1673-5374.257518. Neural Regen Res. 2019. PMID: 31169180 Free PMC article. No abstract available.
-
Perineural growth in head and neck squamous cell carcinoma: a review.Oral Oncol. 2015 Jan;51(1):16-23. doi: 10.1016/j.oraloncology.2014.10.004. Epub 2014 Oct 25. Oral Oncol. 2015. PMID: 25456006 Free PMC article. Review.
-
Causal relationship between circulating cytokines and follicular lymphoma: a two-sample Mendelian randomization study.Am J Cancer Res. 2024 Apr 15;14(4):1577-1593. doi: 10.62347/JCKD6973. eCollection 2024. Am J Cancer Res. 2024. PMID: 38726270 Free PMC article.
-
Trks are novel oncogenes involved in the induction of neovascularization, tumor progression, and nodal metastasis in oral squamous cell carcinoma.Clin Exp Metastasis. 2013 Feb;30(2):165-76. doi: 10.1007/s10585-012-9525-x. Epub 2012 Aug 12. Clin Exp Metastasis. 2013. PMID: 22886570
-
Advances of nanotechnology for intracerebral hemorrhage therapy.Front Bioeng Biotechnol. 2023 Sep 12;11:1265153. doi: 10.3389/fbioe.2023.1265153. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37771570 Free PMC article. Review.
References
-
- Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17(11 Reviews):1343–1352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

