The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes
- PMID: 20159556
- PMCID: PMC2825348
- DOI: 10.1016/j.molcel.2009.12.037
The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes
Abstract
The DENN domain is an evolutionarily ancient protein module. Mutations in the DENN domain cause developmental defects in plants and human diseases, yet the function of this common module is unknown. We now demonstrate that the connecdenn/DENND1A DENN domain functions as a guanine nucleotide exchange factor (GEF) for Rab35 to regulate endosomal membrane trafficking. Loss of Rab35 activity causes an enlargement of early endosomes and inhibits MHC class I recycling. Moreover, it prevents early endosomal recruitment of EHD1, a common component of tubules involved in endosomal cargo recycling. Our data reveal an enzymatic activity for a DENN domain and demonstrate that distinct Rab GTPases can recruit a common protein machinery to various sites within the endosomal network to establish cargo-selective recycling pathways.
Figures
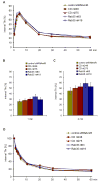
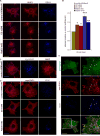
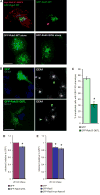
Similar articles
-
The connecdenn family, Rab35 guanine nucleotide exchange factors interfacing with the clathrin machinery.J Biol Chem. 2010 Apr 2;285(14):10627-37. doi: 10.1074/jbc.M109.050930. Epub 2010 Feb 12. J Biol Chem. 2010. PMID: 20154091 Free PMC article.
-
Phosphorylation-dependent Regulation of Connecdenn/DENND1 Guanine Nucleotide Exchange Factors.J Biol Chem. 2015 Jul 17;290(29):17999-18008. doi: 10.1074/jbc.M115.636712. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055712 Free PMC article.
-
Connecdenn 3/DENND1C binds actin linking Rab35 activation to the actin cytoskeleton.Mol Biol Cell. 2012 Jan;23(1):163-75. doi: 10.1091/mbc.E11-05-0474. Epub 2011 Nov 9. Mol Biol Cell. 2012. PMID: 22072793 Free PMC article.
-
Rab35: GEFs, GAPs and effectors.Traffic. 2013 Nov;14(11):1109-17. doi: 10.1111/tra.12096. Epub 2013 Aug 21. Traffic. 2013. PMID: 23905989 Review.
-
DENN domain proteins: regulators of Rab GTPases.J Biol Chem. 2011 Apr 22;286(16):13791-800. doi: 10.1074/jbc.R110.217067. Epub 2011 Feb 17. J Biol Chem. 2011. PMID: 21330364 Free PMC article. Review.
Cited by
-
Rab35 regulates cadherin-mediated adherens junction formation and myoblast fusion.Mol Biol Cell. 2013 Feb;24(3):234-45. doi: 10.1091/mbc.E12-02-0167. Epub 2012 Nov 28. Mol Biol Cell. 2013. PMID: 23197472 Free PMC article.
-
Rab35 regulates skeletogenesis and gastrulation by facilitating actin remodeling and vesicular trafficking.Cells Dev. 2021 Mar;165:203660. doi: 10.1016/j.cdev.2021.203660. Epub 2021 Feb 8. Cells Dev. 2021. PMID: 33842922 Free PMC article.
-
Activity-Dependent Degradation of Synaptic Vesicle Proteins Requires Rab35 and the ESCRT Pathway.J Neurosci. 2016 Aug 17;36(33):8668-86. doi: 10.1523/JNEUROSCI.0725-16.2016. J Neurosci. 2016. PMID: 27535913 Free PMC article.
-
Regulation of Cancer Cell Behavior by the Small GTPase Rab13.J Biol Chem. 2016 May 6;291(19):9929-37. doi: 10.1074/jbc.R116.715193. Epub 2016 Apr 4. J Biol Chem. 2016. PMID: 27044746 Free PMC article. Review.
-
Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells.Dev Cell. 2012 May 15;22(5):952-66. doi: 10.1016/j.devcel.2012.04.010. Dev Cell. 2012. PMID: 22595670 Free PMC article.
References
-
- Allaire PD, Ritter B, Thomas S, Burman JL, Denisov AY, Legendre-Guillemin V, Harper SQ, Davidson BL, Gehring K, McPherson PS. Connecdenn, a novel DENN domain-containing protein of neuronal clathrin-coated vesicles functioning in synaptic vesicle endocytosis. J Neurosci. 2006;26:13202–13212. - PMC - PubMed
-
- Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, et al. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72:1141–1153. - PMC - PubMed
-
- Blume JJ, Halbach A, Behrendt D, Paulsson M, Plomann M. EHD proteins are associated with tubular and vesicular compartments and interact with specific phospholipids. Exp Cell Res. 2007;313:219–231. - PubMed
-
- Chevallier J, Koop C, Srivastava A, Petrie RJ, Lamarche-Vane N, Presley JF. Rab35 regulates neurite outgrowth and cell shape. FEBS Lett. 2009;583:1096–1101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

