Narrative review: the role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes
- PMID: 20157138
- PMCID: PMC2846792
- DOI: 10.7326/0003-4819-152-4-201002160-00008
Narrative review: the role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes
Abstract
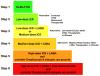
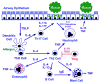
New therapeutic approaches are needed for patients with severe asthma who are refractory to standard therapy comprising high doses of inhaled corticosteroids plus long-acting beta(2)-agonists. Current treatment guidelines for patients with severe asthma from the National Asthma Education and Prevention Program recommend the addition of oral corticosteroids, which are associated with substantial morbidity, and, for those with allergic asthma, anti-IgE. Genetic and translational studies, as well as clinical trials, suggest that in a subgroup of patients, the pathobiology of severe asthma is mediated by immune pathways driven by T-helper 2 (Th2)-type CD4(+) T cells, which produce a characteristic repertoire of interleukins (ILs), including IL-4, IL-5, and IL-13. Therefore, biological modifiers of Th2-type ILs, such as monoclonal antibodies, soluble receptors, and receptor antagonists, are a rational strategy for developing new treatment approaches but will need to be targeted to selected patients in whom the appropriate Th2 immune pathway is "active." The benefits of immune-modifier therapies targeting Th2-type cytokines, however, need to be weighed against the toxicities associated with inhibition of key biological pathways, as well as the expense of future medications. Therefore, future clinical trials need to clearly establish the efficacy and safety of biological modifiers of Th2 immune pathways before these approaches can enter routine clinical practice for the treatment of severe asthma.
Conflict of interest statement
Dr. Wenzel has received consulting fees from Centocor and was Chair of the Steering Committee for the Anti-TNF trial in severe asthma. She has received consulting fees from Genentech and Novartis in relation to treatment of severe asthma. She has consulted for GlaxoSmithKline, Wyeth, Amgen, and Altair. She serves on the Scientific Advisory Board for Altair. Dr. Wenzel has received grants from GlaxoSmithKline, Ception, Medimmune and Aerovance.
Figures
Similar articles
-
Asthma phenotyping: a necessity for improved therapeutic precision and new targeted therapies.J Intern Med. 2016 Feb;279(2):192-204. doi: 10.1111/joim.12382. Epub 2015 Jun 15. J Intern Med. 2016. PMID: 26076339 Review.
-
Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care.J Allergy Clin Immunol. 2015 Feb;135(2):299-310; quiz 311. doi: 10.1016/j.jaci.2014.12.1871. J Allergy Clin Immunol. 2015. PMID: 25662302 Review.
-
Th2 cytokine antagonists: potential treatments for severe asthma.Expert Opin Investig Drugs. 2013 Jan;22(1):49-69. doi: 10.1517/13543784.2013.732997. Epub 2012 Nov 5. Expert Opin Investig Drugs. 2013. PMID: 23126660 Review.
-
Recent changes in the drug treatment of allergic asthma.Clin Med (Lond). 2013 Oct;13(5):477-81. doi: 10.7861/clinmedicine.13-5-477. Clin Med (Lond). 2013. PMID: 24115705 Free PMC article. Review.
-
Targeting the interleukin pathway in the treatment of asthma.Lancet. 2015 Sep 12;386(9998):1086-96. doi: 10.1016/S0140-6736(15)00157-9. Lancet. 2015. PMID: 26383000 Review.
Cited by
-
The critical role of complement alternative pathway regulator factor H in allergen-induced airway hyperresponsiveness and inflammation.J Immunol. 2012 Jan 15;188(2):661-7. doi: 10.4049/jimmunol.1101813. Epub 2011 Dec 14. J Immunol. 2012. PMID: 22174452 Free PMC article.
-
T helper 2 differentiation is necessary for development of lymphedema.Transl Res. 2019 Apr;206:57-70. doi: 10.1016/j.trsl.2018.12.003. Epub 2018 Dec 21. Transl Res. 2019. PMID: 30633890 Free PMC article.
-
Burn wound γδ T-cells support a Th2 and Th17 immune response.J Burn Care Res. 2014 Jan-Feb;35(1):46-53. doi: 10.1097/01.bcr.0000440705.91099.cc. J Burn Care Res. 2014. PMID: 24270084 Free PMC article.
-
Allergen provocation tests in respiratory research: building on 50 years of experience.Eur Respir J. 2022 Aug 25;60(2):2102782. doi: 10.1183/13993003.02782-2021. Print 2022 Aug. Eur Respir J. 2022. PMID: 35086834 Free PMC article. Review.
-
A murine model of peanut-allergic asthma.Front Allergy. 2024 Apr 25;5:1378877. doi: 10.3389/falgy.2024.1378877. eCollection 2024. Front Allergy. 2024. PMID: 38765484 Free PMC article.
References
-
- Am J Respir Crit Care Med; Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions; American Thoracic Society; 2000. pp. 2341–51. - PubMed
-
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–44. - PubMed
-
- Expert Panel Report 3: Guidelines for the Diagnosis and Managment of Asthma. National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services; 2007.
-
- Sorkness RL, Bleecker ER, Busse WW, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104(2):394–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
