The structure of the catalytic subunit FANCL of the Fanconi anemia core complex
- PMID: 20154706
- PMCID: PMC2929457
- DOI: 10.1038/nsmb.1759
The structure of the catalytic subunit FANCL of the Fanconi anemia core complex
Abstract
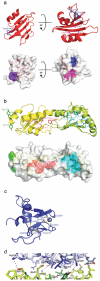
The Fanconi anemia (FA) pathway is activated in response to DNA damage, leading to monoubiquitination of the substrates FANCI and FANCD2 by the FA core complex. Here we report the crystal structure of FANCL, the catalytic subunit of the FA core complex, at 3.2 A. The structure reveals an architecture fundamentally different from previous sequence-based predictions. The molecule is composed of an N-terminal E2-like fold, which we term the ELF domain, a novel double-RWD (DRWD) domain, and a C-terminal really interesting new gene (RING) domain predicted to facilitate E2 binding. Binding assays show that the DRWD domain, but not the ELF domain, is responsible for substrate binding.
Figures


Similar articles
-
The Fanconi Anemia DNA Repair Pathway Is Regulated by an Interaction between Ubiquitin and the E2-like Fold Domain of FANCL.J Biol Chem. 2015 Aug 21;290(34):20995-21006. doi: 10.1074/jbc.M115.675835. Epub 2015 Jul 6. J Biol Chem. 2015. PMID: 26149689 Free PMC article.
-
Mechanistic insight into site-restricted monoubiquitination of FANCD2 by Ube2t, FANCL, and FANCI.Mol Cell. 2008 Dec 26;32(6):767-77. doi: 10.1016/j.molcel.2008.12.003. Mol Cell. 2008. PMID: 19111657
-
Structural analysis of human FANCL, the E3 ligase in the Fanconi anemia pathway.J Biol Chem. 2011 Sep 16;286(37):32628-37. doi: 10.1074/jbc.M111.244632. Epub 2011 Jul 20. J Biol Chem. 2011. PMID: 21775430 Free PMC article.
-
The Fanconi anemia ID2 complex: dueling saxes at the crossroads.Cell Cycle. 2014;13(19):2999-3015. doi: 10.4161/15384101.2014.956475. Cell Cycle. 2014. PMID: 25486561 Free PMC article. Review.
-
Structural insight into FANCI-FANCD2 monoubiquitination.Essays Biochem. 2020 Oct 26;64(5):807-817. doi: 10.1042/EBC20200001. Essays Biochem. 2020. PMID: 32725171 Free PMC article. Review.
Cited by
-
Massively parallel sequencing, aCGH, and RNA-Seq technologies provide a comprehensive molecular diagnosis of Fanconi anemia.Blood. 2013 May 30;121(22):e138-48. doi: 10.1182/blood-2012-12-474585. Epub 2013 Apr 23. Blood. 2013. PMID: 23613520 Free PMC article.
-
A novel interplay between the Fanconi anemia core complex and ATR-ATRIP kinase during DNA cross-link repair.Nucleic Acids Res. 2013 Aug;41(14):6930-41. doi: 10.1093/nar/gkt467. Epub 2013 May 30. Nucleic Acids Res. 2013. PMID: 23723247 Free PMC article.
-
Mechanism and disease association of E2-conjugating enzymes: lessons from UBE2T and UBE2L3.Biochem J. 2016 Oct 15;473(20):3401-3419. doi: 10.1042/BCJ20160028. Biochem J. 2016. PMID: 27729585 Free PMC article. Review.
-
The Fanconi anaemia components UBE2T and FANCM are functionally linked to nucleotide excision repair.PLoS One. 2012;7(5):e36970. doi: 10.1371/journal.pone.0036970. Epub 2012 May 15. PLoS One. 2012. PMID: 22615860 Free PMC article.
-
The Simple Chordate Ciona intestinalis Has a Reduced Complement of Genes Associated with Fanconi Anemia.Evol Bioinform Online. 2016 Jun 6;12:133-48. doi: 10.4137/EBO.S37920. eCollection 2016. Evol Bioinform Online. 2016. PMID: 27279728 Free PMC article.
References
-
- Alter BP. Fanconi's anemia and malignancies. Am J Hematol. 1996;53:99–110. - PubMed
-
- Howlett NG, et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. - PubMed
-
- Strathdee CA, Duncan AM, Buchwald M. Evidence for at least four Fanconi anaemia genes including FACC on chromosome 9. Nat Genet. 1992;1:196–198. - PubMed
-
- Lo Ten Foe JR, et al. Expression cloning of a cDNA for the major Fanconi anaemia gene, FAA. Nat Genet. 1996;14:320–323. - PubMed
-
- Meetei AR, et al. X-linked inheritance of Fanconi anemia complementation group B. Nat Genet. 2004;36:1219–1224. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

