OGFOD1, a novel modulator of eukaryotic translation initiation factor 2alpha phosphorylation and the cellular response to stress
- PMID: 20154146
- PMCID: PMC2849474
- DOI: 10.1128/MCB.01350-09
OGFOD1, a novel modulator of eukaryotic translation initiation factor 2alpha phosphorylation and the cellular response to stress
Abstract
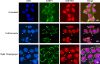
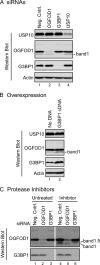
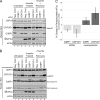
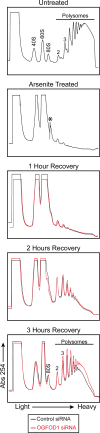
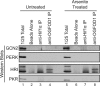
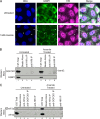
Cells possess mechanisms that permit survival and recovery from stress, several of which regulate the phosphorylation of eukaryotic translation initiation factor 2alpha (eIF2alpha). We identified the human OGFOD1 protein as a novel stress granule component that regulates the phosphorylation of eIF2alpha and the resumption of translation in cells recovering from arsenite-induced stress. Coimmunoprecipitation studies revealed that OGFOD1 associates with a small subset of stress granule proteins (G3BP1, USP10, Caprin1, and YB-1) and the ribosome in both unstressed and stressed cells. Overexpression of OGFOD1 led to increased abundance of phosphorylated eIF2alpha, both in unstressed cells and in cells exposed to arsenite-induced stress, and to accelerated apoptosis during stress. Conversely, knockdown of OGFOD1 resulted in smaller amounts of phosphorylated eIF2alpha and a faster accumulation of polyribosomes in cells recovering from stress. Finally, OGFOD1 interacted with both eIF2alpha and the eIF2alpha kinase heme-regulated inhibitor (HRI), which was identified as a novel stress granule resident. These findings argue that OGFOD1 plays important proapoptotic roles in the regulation of translation and HRI-mediated phosphorylation of eIF2alpha in cells subjected to arsenite-induced stress.
Figures
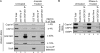
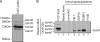

Similar articles
-
G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits.J Cell Biol. 2016 Mar 28;212(7):845-60. doi: 10.1083/jcb.201508028. J Cell Biol. 2016. PMID: 27022092 Free PMC article.
-
Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation.Mol Biol Cell. 2006 Oct;17(10):4212-9. doi: 10.1091/mbc.e06-04-0318. Epub 2006 Jul 26. Mol Biol Cell. 2006. PMID: 16870703 Free PMC article.
-
Large G3BP-induced granules trigger eIF2α phosphorylation.Mol Biol Cell. 2012 Sep;23(18):3499-510. doi: 10.1091/mbc.E12-05-0385. Epub 2012 Jul 25. Mol Biol Cell. 2012. PMID: 22833567 Free PMC article.
-
Mouse Norovirus Infection Arrests Host Cell Translation Uncoupled from the Stress Granule-PKR-eIF2α Axis.mBio. 2019 Jun 18;10(3):e00960-19. doi: 10.1128/mBio.00960-19. mBio. 2019. PMID: 31213553 Free PMC article.
-
The eIF2α kinase HRI in innate immunity, proteostasis, and mitochondrial stress.FEBS J. 2021 May;288(10):3094-3107. doi: 10.1111/febs.15553. Epub 2020 Oct 3. FEBS J. 2021. PMID: 32892501 Review.
Cited by
-
Fe(2)OG: an integrated HMM profile-based web server to predict and analyze putative non-haem iron(II)- and 2-oxoglutarate-dependent dioxygenase function in protein sequences.BMC Res Notes. 2021 Mar 1;14(1):80. doi: 10.1186/s13104-021-05477-z. BMC Res Notes. 2021. PMID: 33648553 Free PMC article.
-
Hepatitis C virus subverts liver-specific miR-122 to protect the viral genome from exoribonuclease Xrn2.Cell Host Microbe. 2014 Aug 13;16(2):257-264. doi: 10.1016/j.chom.2014.07.006. Cell Host Microbe. 2014. PMID: 25121753 Free PMC article.
-
Tracing the dynamics of gene transcripts after organismal death.Open Biol. 2017 Jan;7(1):160267. doi: 10.1098/rsob.160267. Open Biol. 2017. PMID: 28123054 Free PMC article.
-
Co-operative intermolecular kinetics of 2-oxoglutarate dependent dioxygenases may be essential for system-level regulation of plant cell physiology.Front Plant Sci. 2015 Jul 15;6:489. doi: 10.3389/fpls.2015.00489. eCollection 2015. Front Plant Sci. 2015. PMID: 26236316 Free PMC article.
-
An exome study of Parkinson's disease in Sardinia, a Mediterranean genetic isolate.Neurogenetics. 2015 Jan;16(1):55-64. doi: 10.1007/s10048-014-0425-x. Epub 2014 Oct 8. Neurogenetics. 2015. PMID: 25294124
References
-
- Anderson, P., and N. Kedersha. 2009. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 10:430-436. - PubMed
-
- Anderson, P., and N. Kedersha. 2008. Stress granules: the tao of RNA triage. Trends Biochem. Sci. 33:141-150. - PubMed
-
- Arimoto, K., H. Fukuda, S. Imajoh-Ohmi, H. Saito, and M. Takekawa. 2008. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 10:1324-1332. - PubMed
-
- Boyce, M., K. F. Bryant, C. Jousse, K. Long, H. P. Harding, D. Scheuner, R. J. Kaufman, D. Ma, D. M. Coen, D. Ron, and J. Yuan. 2005. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307:935-939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
