Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease
- PMID: 20153282
- PMCID: PMC2824513
- DOI: 10.1016/j.chom.2010.01.004
Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease
Abstract
Dengue virus (DENV) causes disease ranging from dengue fever (DF), a self-limited febrile illness, to the potentially lethal dengue hemorrhagic fever and dengue shock syndrome (DHF/DSS). DHF/DSS usually occurs in patients who have acquired DENV-reactive antibodies prior to infection, either from a previous infection with a heterologous DENV serotype or from an immune mother. Hence, it has been hypothesized that subneutralizing levels of antibodies exacerbate disease, a phenomenon termed antibody-dependent enhancement (ADE). However, given the lack of suitable animal models for DENV infection, the mechanism of ADE and its contribution to pathology remain elusive. Here we demonstrate in mice that DENV-specific antibodies can sufficiently increase severity of disease so that a mostly nonlethal illness becomes a fatal disease resembling human DHF/DSS. Antibodies promote massive infection of liver sinusoidal endothelial cells (LSECs), resulting in increased systemic levels of virus. Thus, a subprotective humoral response may, under some circumstances, have pathological consequences.
2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

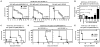

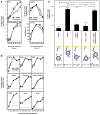
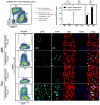
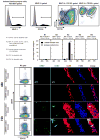
Comment in
-
Modeling antibody-enhanced dengue virus infection and disease in mice: protection or pathogenesis?Cell Host Microbe. 2010 Feb 18;7(2):85-6. doi: 10.1016/j.chom.2010.02.004. Cell Host Microbe. 2010. PMID: 20159612
Similar articles
-
A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model.PLoS Med. 2009 Oct;6(10):e1000171. doi: 10.1371/journal.pmed.1000171. Epub 2009 Oct 27. PLoS Med. 2009. PMID: 19859541 Free PMC article. Clinical Trial.
-
Low levels of antibody-dependent enhancement in vitro using viruses and plasma from dengue patients.PLoS One. 2014 Mar 18;9(3):e92173. doi: 10.1371/journal.pone.0092173. eCollection 2014. PLoS One. 2014. PMID: 24642752 Free PMC article.
-
Progress towards understanding the pathogenesis of dengue hemorrhagic fever.Virol Sin. 2017 Feb;32(1):16-22. doi: 10.1007/s12250-016-3855-9. Epub 2016 Nov 14. Virol Sin. 2017. PMID: 27853992 Free PMC article. Review.
-
Subversion of early innate antiviral responses during antibody-dependent enhancement of Dengue virus infection induces severe disease in immunocompetent mice.Med Microbiol Immunol. 2014 Aug;203(4):231-50. doi: 10.1007/s00430-014-0334-5. Epub 2014 Apr 11. Med Microbiol Immunol. 2014. PMID: 24723052
-
Re-evaluation of the pathogenic roles of nonstructural protein 1 and its antibodies during dengue virus infection.J Biomed Sci. 2013 Jun 27;20(1):42. doi: 10.1186/1423-0127-20-42. J Biomed Sci. 2013. PMID: 23806052 Free PMC article. Review.
Cited by
-
Liver transcriptomics reveals features of the host response in a mouse model of dengue virus infection.Front Immunol. 2022 Aug 26;13:892469. doi: 10.3389/fimmu.2022.892469. eCollection 2022. Front Immunol. 2022. PMID: 36091000 Free PMC article.
-
Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells.J Immunol. 2012 Jan 1;188(1):404-16. doi: 10.4049/jimmunol.1102124. Epub 2011 Nov 30. J Immunol. 2012. PMID: 22131327 Free PMC article.
-
Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis.Nat Rev Microbiol. 2013 Jun;11(6):420-6. doi: 10.1038/nrmicro3030. Epub 2013 May 8. Nat Rev Microbiol. 2013. PMID: 23652323 Review.
-
CD8+ T Cells Can Mediate Short-Term Protection against Heterotypic Dengue Virus Reinfection in Mice.J Virol. 2015 Jun;89(12):6494-505. doi: 10.1128/JVI.00036-15. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855749 Free PMC article.
-
Inhibition of dengue virus infections in cell cultures and in AG129 mice by a small interfering RNA targeting a highly conserved sequence.J Virol. 2011 Oct;85(19):10154-66. doi: 10.1128/JVI.05298-11. Epub 2011 Jul 27. J Virol. 2011. PMID: 21795337 Free PMC article.
References
-
- Balsitis SJ, Coloma J, Castro G, Alava A, Flores D, McKerrow JH, Beatty PR, Harris E. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. The American journal of tropical medicine and hygiene. 2009;80:416–424. - PubMed
-
- Bashirova AA, Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ, Eilering JB, Martin MP, Wu L, Martin TD, Viebig N, Knolle PA, et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. The Journal of experimental medicine. 2001;193:671–678. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

