Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis
- PMID: 20152126
- PMCID: PMC2902371
- DOI: 10.1016/j.neuron.2010.01.011
Hook3 interacts with PCM1 to regulate pericentriolar material assembly and the timing of neurogenesis
Abstract
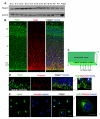
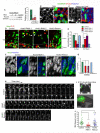
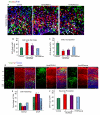
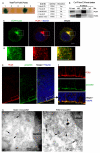
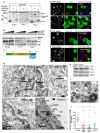
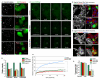
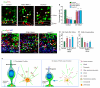
Centrosome functions are important in multiple brain developmental processes. Proper functioning of the centrosome relies on assembly of protein components into the pericentriolar material. This dynamic assembly is mediated by the trafficking of pericentriolar satellites, which are comprised of centrosomal proteins. Here we demonstrate that trafficking of pericentriolar satellites requires the interaction between Hook3 and Pericentriolar Material 1 (PCM1). Hook3, previously shown to link the centrosome and the nucleus in C. elegans, is recruited to pericentriolar satellites through interaction with PCM1, a protein associated with schizophrenia. Disruption of the Hook3-PCM1 interaction in vivo impairs interkinetic nuclear migration, a featured behavior of embryonic neural progenitors. This in turn leads to overproduction of neurons and premature depletion of the neural progenitor pool in the developing neocortex. These results underscore the importance of centrosomal assembly in neurogenesis and provide potential insights into the etiology of brain developmental diseases related to the centrosome dysfunction.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
PCM1 recruits Plk1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry.J Cell Sci. 2013 Mar 15;126(Pt 6):1355-65. doi: 10.1242/jcs.114918. Epub 2013 Jan 23. J Cell Sci. 2013. PMID: 23345402
-
SDCCAG8 regulates pericentriolar material recruitment and neuronal migration in the developing cortex.Neuron. 2014 Aug 20;83(4):805-22. doi: 10.1016/j.neuron.2014.06.029. Epub 2014 Jul 31. Neuron. 2014. PMID: 25088364 Free PMC article.
-
Zika virus increases mind bomb 1 levels, causing degradation of pericentriolar material 1 (PCM1) and dispersion of PCM1-containing granules from the centrosome.J Biol Chem. 2019 Dec 6;294(49):18742-18755. doi: 10.1074/jbc.RA119.010973. Epub 2019 Oct 30. J Biol Chem. 2019. PMID: 31666336 Free PMC article.
-
Pericentriolar material structure and dynamics.Philos Trans R Soc Lond B Biol Sci. 2014 Sep 5;369(1650):20130459. doi: 10.1098/rstb.2013.0459. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25047613 Free PMC article. Review.
-
[Centrosomal proteins].Biokhimiia. 1996 Aug;61(8):1347-65. Biokhimiia. 1996. PMID: 8962912 Review. Russian.
Cited by
-
Cell biological regulation of division fate in vertebrate neuroepithelial cells.Dev Dyn. 2011 Aug;240(8):1865-79. doi: 10.1002/dvdy.22684. Dev Dyn. 2011. PMID: 21761474 Free PMC article. Review.
-
PCM1 is necessary for focal ciliary integrity and is a candidate for severe schizophrenia.Nat Commun. 2020 Nov 19;11(1):5903. doi: 10.1038/s41467-020-19637-5. Nat Commun. 2020. PMID: 33214552 Free PMC article.
-
Dynamic Polarization of Rab11a Modulates Crb2a Localization and Impacts Signaling to Regulate Retinal Neurogenesis.Front Cell Dev Biol. 2021 Feb 9;8:608112. doi: 10.3389/fcell.2020.608112. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33634099 Free PMC article.
-
DISC1: a key lead in studying cortical development and associated brain disorders.Neuroscientist. 2013 Oct;19(5):451-64. doi: 10.1177/1073858412470168. Epub 2013 Jan 8. Neuroscientist. 2013. PMID: 23300216 Free PMC article. Review.
-
High-Level HOOK3 Expression Is an Independent Predictor of Poor Prognosis Associated with Genomic Instability in Prostate Cancer.PLoS One. 2015 Jul 31;10(7):e0134614. doi: 10.1371/journal.pone.0134614. eCollection 2015. PLoS One. 2015. PMID: 26230842 Free PMC article.
References
-
- Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. - PubMed
-
- Baron AT, Suman VJ, Nemeth E, Salisbury JL. The pericentriolar lattice of PtK2 cells exhibits temperature and calcium-modulated behavior. J Cell Sci. 1994;107(Pt 11):2993–3003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

