Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL)
- PMID: 20150572
- PMCID: PMC2924992
- DOI: 10.1093/annonc/mdq020
Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL)
Abstract
Background: Bevacizumab, the anti-vascular endothelial growth factor agent, provides clinical benefit when combined with platinum-based chemotherapy in first-line advanced non-small-cell lung cancer. We report the final overall survival (OS) analysis from the phase III AVAiL trial.
Patients and methods: Patients (n = 1043) received cisplatin 80 mg/m(2) and gemcitabine 1250 mg/m(2) for up to six cycles plus bevacizumab 7.5 mg/kg (n = 345), bevacizumab 15 mg/kg (n = 351) or placebo (n = 347) every 3 weeks until progression. Primary end point was progression-free survival (PFS); OS was a secondary end point.
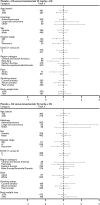
Results: Significant PFS prolongation with bevacizumab compared with placebo was maintained with longer follow-up {hazard ratio (HR) [95% confidence interval (CI)] 0.75 (0.64-0.87), P = 0.0003 and 0.85 (0.73-1.00), P = 0.0456} for the 7.5 and 15 mg/kg groups, respectively. Median OS was >13 months in all treatment groups; nevertheless, OS was not significantly increased with bevacizumab [HR (95% CI) 0.93 (0.78-1.11), P = 0.420 and 1.03 (0.86-1.23), P = 0.761] for the 7.5 and 15 mg/kg groups, respectively, versus placebo. Most patients ( approximately 62%) received multiple lines of poststudy treatment. Updated safety results are consistent with those previously reported.
Conclusions: Final analysis of AVAiL confirms the efficacy of bevacizumab when combined with cisplatin-gemcitabine. The PFS benefit did not translate into a significant OS benefit, possibly due to high use of efficacious second-line therapies.
Trial registration: ClinicalTrials.gov NCT00806923.
Figures

Similar articles
-
Efficacy and safety of bevacizumab-based therapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer in the phase III BO17704 study (AVAiL).J Thorac Oncol. 2010 Dec;5(12):1970-6. doi: 10.1097/JTO.0b013e3181f49c22. J Thorac Oncol. 2010. PMID: 20978447 Clinical Trial.
-
Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil.J Clin Oncol. 2009 Mar 10;27(8):1227-34. doi: 10.1200/JCO.2007.14.5466. Epub 2009 Feb 2. J Clin Oncol. 2009. PMID: 19188680 Clinical Trial.
-
Efficacy of bevacizumab with cisplatin and gemcitabine in Asian patients with advanced or recurrent non-squamous non-small cell lung cancer who have not received prior chemotherapy: a substudy of the Avastin in Lung trial.Asia Pac J Clin Oncol. 2011 Jun;7 Suppl 2:4-12. doi: 10.1111/j.1743-7563.2011.01397.x. Asia Pac J Clin Oncol. 2011. PMID: 21585703 Clinical Trial.
-
Antibodies to vascular endothelial growth factor in non-small cell lung cancer.J Thorac Oncol. 2008 Jun;3(6 Suppl 2):S113-8. doi: 10.1097/JTO.0b013e318174e993. J Thorac Oncol. 2008. PMID: 18520292 Review.
-
Bevacizumab in non-small cell lung cancer.Drugs. 2008;68(6):737-46. doi: 10.2165/00003495-200868060-00002. Drugs. 2008. PMID: 18416583 Review.
Cited by
-
The impact of Bevacizumab (Avastin) on survival in metastatic solid tumors--a meta-analysis and systematic review.PLoS One. 2013;8(1):e51780. doi: 10.1371/journal.pone.0051780. Epub 2013 Jan 22. PLoS One. 2013. PMID: 23349675 Free PMC article. Review.
-
Same Data; Different Interpretations.J Clin Oncol. 2016 Nov 1;34(31):3729-3732. doi: 10.1200/JCO.2016.68.2021. J Clin Oncol. 2016. PMID: 27573659 Free PMC article. No abstract available.
-
Anti-HDGF targets cancer and cancer stromal stem cells resistant to chemotherapy.Clin Cancer Res. 2013 Jul 1;19(13):3567-76. doi: 10.1158/1078-0432.CCR-12-3478. Epub 2013 May 21. Clin Cancer Res. 2013. PMID: 23695169 Free PMC article.
-
KRAS Mutations Predict Response and Outcome in Advanced Lung Adenocarcinoma Patients Receiving First-Line Bevacizumab and Platinum-Based Chemotherapy.Cancers (Basel). 2019 Oct 9;11(10):1514. doi: 10.3390/cancers11101514. Cancers (Basel). 2019. PMID: 31600989 Free PMC article.
-
Clinical Outcomes of Chemotherapeutic Molecules as Single and Multiple Agents in Advanced Non-Small-Cell Lung Carcinoma (NSCLC) Patients.Medicina (Kaunas). 2021 Nov 16;57(11):1252. doi: 10.3390/medicina57111252. Medicina (Kaunas). 2021. PMID: 34833470 Free PMC article. Review.
References
-
- Smit EF, van Meerbeeck JP, Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group—EORTC 08975. J Clin Oncol. 2003;21:3909–3917. - PubMed
-
- Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. - PubMed
-
- Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. - PubMed
-
- Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

