CCR6 identifies lymphoid tissue inducer cells within cryptopatches
- PMID: 20148914
- PMCID: PMC2883115
- DOI: 10.1111/j.1365-2249.2010.04103.x
CCR6 identifies lymphoid tissue inducer cells within cryptopatches
Abstract
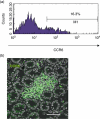
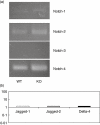
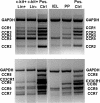
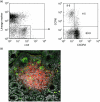
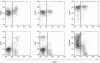
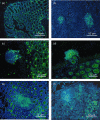
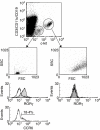
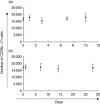
The chemokine receptor CCR6 is expressed by dendritic cells, B and T cells predominantly within the organized structures of the gut-associated lymphatic tissue. Its ligand CCL20 is synthesized by the follicle-associated epithelium and is crucial for the development of M cells within Peyer's patches. In addition, lineage-negative c-kit positive lymphocytes within cryptopatches (CP) express CCR6. CCR6-deficient mice exhibit an altered intestinal immune system containing increased amounts of intraepithelial lymphocytes and show smaller Peyer's patches, while progression of cryptopatches to mature isolated lymphoid follicles (ILF) is inhibited. In this report, we show that lin(-) c-kit(+) lymphocytes express a variety of different chemokine receptors and that CCR6 identifies those cells located within CP. In contrast, cells found outside CP are positive for CXCR3 and exhibit a different surface marker profile, suggesting that at least two different populations of lin(-) c-kit(+) cells are present. The presence of CCR6 does not influence the expression of Notch molecules on lin(-) c-kit(+) cells, nor does it influence Notch ligand expression on bone marrow-derived dendritic cells. In the human gut, CCR6 identifies clusters of lymphocytes resembling murine CP. CCR6 seems to have an important role for lin(-) c-kit(+) cells inside CP, is expressed in a regulated manner and identifies potential human CP.
Figures
Similar articles
-
Lymphoid precursors in intestinal cryptopatches express CCR6 and undergo dysregulated development in the absence of CCR6.J Immunol. 2003 Sep 1;171(5):2208-15. doi: 10.4049/jimmunol.171.5.2208. J Immunol. 2003. PMID: 12928364
-
Induction of intestinal lymphoid tissue: the role of cryptopatches.Ann N Y Acad Sci. 2006 Aug;1072:210-7. doi: 10.1196/annals.1326.015. Ann N Y Acad Sci. 2006. PMID: 17057201 Review.
-
CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis.Ann N Y Acad Sci. 2006 Aug;1072:52-61. doi: 10.1196/annals.1326.036. Ann N Y Acad Sci. 2006. PMID: 17057190 Review.
-
CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles.Am J Pathol. 2007 Apr;170(4):1229-40. doi: 10.2353/ajpath.2007.060817. Am J Pathol. 2007. PMID: 17392163 Free PMC article.
-
CCR6hiCD11c(int) B cells promote M-cell differentiation in Peyer's patch.Int Immunol. 2011 Apr;23(4):261-9. doi: 10.1093/intimm/dxq478. Epub 2011 Mar 21. Int Immunol. 2011. PMID: 21422150 Free PMC article.
Cited by
-
Cryptopatches are essential for the development of human GALT.Cell Rep. 2013 Jun 27;3(6):1874-84. doi: 10.1016/j.celrep.2013.05.037. Epub 2013 Jun 20. Cell Rep. 2013. PMID: 23791525 Free PMC article.
-
Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier.Nature. 2020 Mar;579(7800):575-580. doi: 10.1038/s41586-020-2039-9. Epub 2020 Feb 12. Nature. 2020. PMID: 32050257 Free PMC article.
-
The Development of Steady-State Activation Hubs between Adult LTi ILC3s and Primed Macrophages in Small Intestine.J Immunol. 2017 Sep 1;199(5):1912-1922. doi: 10.4049/jimmunol.1700155. Epub 2017 Jul 26. J Immunol. 2017. PMID: 28747343 Free PMC article.
-
Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury.Front Immunol. 2020 Aug 4;11:1722. doi: 10.3389/fimmu.2020.01722. eCollection 2020. Front Immunol. 2020. PMID: 32849610 Free PMC article. Review.
-
Intestinal CD4 Depletion in HIV / SIV Infection.Curr Immunol Rev. 2019;15(1):76-91. doi: 10.2174/1573395514666180605083448. Curr Immunol Rev. 2019. PMID: 31431807 Free PMC article.
References
-
- Taylor RT, Lugering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173:7183–9. - PubMed
-
- Saito H, Kanamori Y, Takemori T, et al. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–8. - PubMed
-
- Suzuki K, Oida T, Hamada H, et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13:691–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

