LnaB: a Legionella pneumophila activator of NF-kappaB
- PMID: 20148897
- PMCID: PMC2947841
- DOI: 10.1111/j.1462-5822.2010.01452.x
LnaB: a Legionella pneumophila activator of NF-kappaB
Abstract
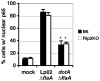
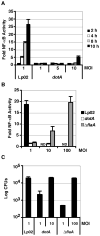
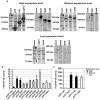
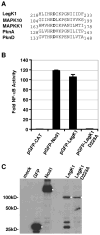
Legionella pneumophila possesses a large arsenal of type IV translocated substrates. Over 100 such proteins have been identified, but the functions of most are unknown. Previous studies have demonstrated that L. pneumophila activates NF-kappaB, a master transcriptional regulator of the mammalian innate immune response. Activation of NF-kappaB is dependent on the Legionella Icm/Dot type IV protein translocation system, consistent with the possibility that translocated bacterial proteins contribute to this response. To test this hypothesis, an expression library of 159 known and putative translocated substrates was created to evaluate whether ectopic production of a single L. pneumophila protein could activate NF-kappaB in mammalian cells. Expression of two of these proteins, LnaB (Legionella NF-kappaB activator B) and LegK1, resulted in approximately 150-fold induction of NF-kappaB activity in HEK293T cells, levels similar to the strong induction that occurs with ectopic expression of the known activator Nod1. LnaB is a substrate of the Icm/Dot system, and in the absence of this protein, a partial reduction of NF-kappaB activation in host cells occurs after challenge by post-exponential phase bacteria. These data indicate that LnaB is an Icm/Dot substrate that contributes to NF-kappaB activation during L. pneumophila infection in host cells.
Figures

Similar articles
-
Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila.PLoS Pathog. 2011 Feb;7(2):e1001289. doi: 10.1371/journal.ppat.1001289. Epub 2011 Feb 17. PLoS Pathog. 2011. PMID: 21390206 Free PMC article.
-
NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila.J Exp Med. 2006 Sep 4;203(9):2177-89. doi: 10.1084/jem.20060766. Epub 2006 Aug 28. J Exp Med. 2006. PMID: 16940169 Free PMC article.
-
A Legionella type IV effector activates the NF-kappaB pathway by phosphorylating the IkappaB family of inhibitors.Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13725-30. doi: 10.1073/pnas.0907200106. Epub 2009 Aug 3. Proc Natl Acad Sci U S A. 2009. PMID: 19666608 Free PMC article.
-
Legionella pneumophila Dot/Icm translocated substrates: a sum of parts.Curr Opin Microbiol. 2009 Feb;12(1):67-73. doi: 10.1016/j.mib.2008.12.004. Epub 2009 Jan 20. Curr Opin Microbiol. 2009. PMID: 19157961 Free PMC article. Review.
-
Manipulation of host vesicular trafficking and innate immune defence by Legionella Dot/Icm effectors.Cell Microbiol. 2011 Dec;13(12):1870-80. doi: 10.1111/j.1462-5822.2011.01710.x. Epub 2011 Nov 10. Cell Microbiol. 2011. PMID: 21981078 Review.
Cited by
-
Host-pathogen interaction profiling using self-assembling human protein arrays.J Proteome Res. 2015 Apr 3;14(4):1920-36. doi: 10.1021/pr5013015. Epub 2015 Mar 18. J Proteome Res. 2015. PMID: 25739981 Free PMC article.
-
Study of Legionella Effector Domains Revealed Novel and Prevalent Phosphatidylinositol 3-Phosphate Binding Domains.Infect Immun. 2019 May 21;87(6):e00153-19. doi: 10.1128/IAI.00153-19. Print 2019 Jun. Infect Immun. 2019. PMID: 30962397 Free PMC article.
-
Viewing Legionella pneumophila Pathogenesis through an Immunological Lens.J Mol Biol. 2019 Oct 4;431(21):4321-4344. doi: 10.1016/j.jmb.2019.07.028. Epub 2019 Jul 25. J Mol Biol. 2019. PMID: 31351897 Free PMC article. Review.
-
Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia.Infect Immun. 2011 May;79(5):1984-97. doi: 10.1128/IAI.01077-10. Epub 2011 Mar 7. Infect Immun. 2011. PMID: 21383054 Free PMC article.
-
Legionella pneumophila effector Lem4 is a membrane-associated protein tyrosine phosphatase.J Biol Chem. 2018 Aug 24;293(34):13044-13058. doi: 10.1074/jbc.RA118.003845. Epub 2018 Jul 5. J Biol Chem. 2018. PMID: 29976756 Free PMC article.
References
-
- Abu-Zant A, Jones S, Asare R, Suttles J, Price C, Graham J, Kwaik YA. Anti-apoptotic signalling by the Dot/Icm secretion system of L. pneumophila. Cell Microbiol. 2007;9:246–264. - PubMed
-
- Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, Jungblut PR, et al. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2:155–164. - PubMed
-
- Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. - PubMed
-
- Bartfeld S, Engels C, Bauer B, Aurass P, Flieger A, Bruggemann H, Meyer TF. Temporal resolution of two-tracked NF-κB activation by Legionella pneumophila. Cell Microbiol. 2009;11:1638–1651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

