Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease
- PMID: 20147699
- PMCID: PMC2858477
- DOI: 10.1182/blood-2009-10-245225
Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease
Abstract
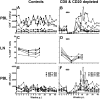
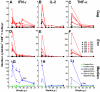
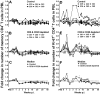
One of the most puzzling observations in HIV research is the lack of pathogenicity in most nonhuman primate species that are natural hosts of simian immunodeficiency virus (SIV) infection. Despite this, natural hosts experience a level of viremia similar to humans infected with HIV or macaques infected with SIV. To determine the role of adaptive immune responses in viral containment and lack of disease, we delayed the generation of cellular and humoral immune responses by administering anti-CD8- and anti-CD20 lymphocyte-depleting antibodies to sabaeus African green monkeys (Chlorocebus sabaeus) before challenge with SIV(sab9315BR). In vivo lymphocyte depletion during primary infection resulted in a brief elevation of viremia but not in disease. Based on the magnitude and timing of SIV-specific CD8(+) T-cell responses in the lymphocyte-depleted animals, CD8(+) T-cell responses appear to contribute to viral containment in natural hosts. We found no evidence for a contribution of humoral immune responses in viral containment. These studies indicate that natural hosts have developed mechanisms in addition to classic adaptive immune responses to cope with this lentiviral infection. Thus, adaptive immune responses in natural hosts appear to be less critical for viral containment than in HIV infection.
Figures




Similar articles
-
Inhibition of adaptive immune responses leads to a fatal clinical outcome in SIV-infected pigtailed macaques but not vervet African green monkeys.PLoS Pathog. 2009 Dec;5(12):e1000691. doi: 10.1371/journal.ppat.1000691. Epub 2009 Dec 11. PLoS Pathog. 2009. PMID: 20011508 Free PMC article.
-
Emergence of simian immunodeficiency virus-specific cytotoxic CD4+ T cells and increased humoral responses correlate with control of rebounding viremia in CD8-depleted macaques infected with Rev-independent live-attenuated simian immunodeficiency virus.J Immunol. 2010 Sep 15;185(6):3348-58. doi: 10.4049/jimmunol.1000572. Epub 2010 Aug 11. J Immunol. 2010. PMID: 20702730 Free PMC article.
-
Effect of B-cell depletion on viral replication and clinical outcome of simian immunodeficiency virus infection in a natural host.J Virol. 2009 Oct;83(20):10347-57. doi: 10.1128/JVI.00880-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656874 Free PMC article.
-
Walk on the wild side: SIV infection in African non-human primate hosts-from the field to the laboratory.Front Immunol. 2023 Jan 12;13:1060985. doi: 10.3389/fimmu.2022.1060985. eCollection 2022. Front Immunol. 2023. PMID: 36713371 Free PMC article. Review.
-
Natural SIV hosts: showing AIDS the door.Science. 2012 Mar 9;335(6073):1188-93. doi: 10.1126/science.1217550. Science. 2012. PMID: 22403383 Free PMC article. Review.
Cited by
-
Characterization of Simian Immunodeficiency Virus Variants Anatomically Compartmentalized in Plasma and Milk in Chronically Infected African Green Monkeys.J Virol. 2016 Sep 12;90(19):8795-808. doi: 10.1128/JVI.00701-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466415 Free PMC article.
-
So Pathogenic or So What?-A Brief Overview of SIV Pathogenesis with an Emphasis on Cure Research.Viruses. 2022 Jan 12;14(1):135. doi: 10.3390/v14010135. Viruses. 2022. PMID: 35062339 Free PMC article. Review.
-
Mucosal simian immunodeficiency virus transmission in African green monkeys: susceptibility to infection is proportional to target cell availability at mucosal sites.J Virol. 2012 Apr;86(8):4158-68. doi: 10.1128/JVI.07141-11. Epub 2012 Feb 8. J Virol. 2012. PMID: 22318138 Free PMC article.
-
Magnitude and Quality of Cytokine and Chemokine Storm during Acute Infection Distinguish Nonprogressive and Progressive Simian Immunodeficiency Virus Infections of Nonhuman Primates.J Virol. 2016 Oct 28;90(22):10339-10350. doi: 10.1128/JVI.01061-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27630228 Free PMC article.
-
Early induction of polyfunctional simian immunodeficiency virus (SIV)-specific T lymphocytes and rapid disappearance of SIV from lymph nodes of sooty mangabeys during primary infection.J Immunol. 2011 May 1;186(9):5151-61. doi: 10.4049/jimmunol.1004110. Epub 2011 Mar 25. J Immunol. 2011. PMID: 21441446 Free PMC article.
References
-
- Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9(7):861–866. - PubMed
-
- Kuroda MJ, Schmitz JE, Charini WA, et al. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162(9):5127–5133. - PubMed
-
- Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01AI30034/AI/NIAID NIH HHS/United States
- RR000168/RR/NCRR NIH HHS/United States
- AI040101/AI/NIAID NIH HHS/United States
- R24 RR016001/RR/NCRR NIH HHS/United States
- AI067854/AI/NIAID NIH HHS/United States
- R01 AI043890/AI/NIAID NIH HHS/United States
- AI43890/AI/NIAID NIH HHS/United States
- RR016001/RR/NCRR NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R01 AI065335/AI/NIAID NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- AI060354/AI/NIAID NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- AI065335/AI/NIAID NIH HHS/United States
- R01 AI040101/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

