Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior
- PMID: 20147539
- PMCID: PMC2824441
- DOI: 10.1523/JNEUROSCI.4517-09.2010
Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior
Abstract
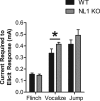
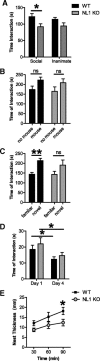
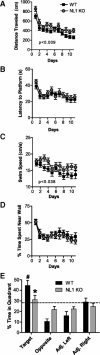
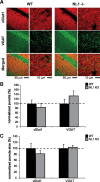
Neuroligins (NLs) are a family of neural cell-adhesion molecules that are involved in excitatory/inhibitory synapse specification. Multiple members of the NL family (including NL1) and their binding partners have been linked to cases of human autism and mental retardation. We have now characterized NL1-deficient mice in autism- and mental retardation-relevant behavioral tasks. NL1 knock-out (KO) mice display deficits in spatial learning and memory that correlate with impaired hippocampal long-term potentiation. In addition, NL1 KO mice exhibit a dramatic increase in repetitive, stereotyped grooming behavior, a potential autism-relevant abnormality. This repetitive grooming abnormality in NL1 KO mice is associated with a reduced NMDA/AMPA ratio at corticostriatal synapses. Interestingly, we further demonstrate that the increased repetitive grooming phenotype can be rescued in adult mice by administration of the NMDA receptor partial coagonist d-cycloserine. Broadly, these data are consistent with a role of synaptic cell-adhesion molecules in general, and NL1 in particular, in autism and implicate reduced excitatory synaptic transmission as a potential mechanism and treatment target for repetitive behavioral abnormalities.
Figures


Similar articles
-
Neuroligins Are Selectively Essential for NMDAR Signaling in Cerebellar Stellate Interneurons.J Neurosci. 2016 Aug 31;36(35):9070-83. doi: 10.1523/JNEUROSCI.1356-16.2016. J Neurosci. 2016. PMID: 27581450 Free PMC article.
-
Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function.Proc Natl Acad Sci U S A. 2011 Aug 16;108(33):13764-9. doi: 10.1073/pnas.1111093108. Epub 2011 Aug 1. Proc Natl Acad Sci U S A. 2011. PMID: 21808020 Free PMC article.
-
Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism.Mol Psychiatry. 2017 Mar;22(3):375-383. doi: 10.1038/mp.2016.80. Epub 2016 May 24. Mol Psychiatry. 2017. PMID: 27217145 Free PMC article.
-
[Synapse maturation and autism: learning from neuroligin model mice].Nihon Shinkei Seishin Yakurigaku Zasshi. 2014 Feb;34(1):1-4. Nihon Shinkei Seishin Yakurigaku Zasshi. 2014. PMID: 25069265 Review. Japanese.
-
A review on the current neuroligin mouse models.Sheng Li Xue Bao. 2012 Oct 25;64(5):550-62. Sheng Li Xue Bao. 2012. PMID: 23090496 Review.
Cited by
-
A subtype-specific function for the extracellular domain of neuroligin 1 in hippocampal LTP.Neuron. 2012 Oct 18;76(2):309-16. doi: 10.1016/j.neuron.2012.07.024. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083734 Free PMC article.
-
Progress toward treatments for synaptic defects in autism.Nat Med. 2013 Jun;19(6):685-94. doi: 10.1038/nm.3193. Epub 2013 Jun 6. Nat Med. 2013. PMID: 23744158 Review.
-
Gephyrin, the enigmatic organizer at GABAergic synapses.Front Cell Neurosci. 2012 May 15;6:23. doi: 10.3389/fncel.2012.00023. eCollection 2012. Front Cell Neurosci. 2012. PMID: 22615685 Free PMC article.
-
Autism-Associated Insertion Mutation (InsG) of Shank3 Exon 21 Causes Impaired Synaptic Transmission and Behavioral Deficits.J Neurosci. 2015 Jul 1;35(26):9648-65. doi: 10.1523/JNEUROSCI.3125-14.2015. J Neurosci. 2015. PMID: 26134648 Free PMC article.
-
The ADHD-linked human dopamine D4 receptor variant D4.7 induces over-suppression of NMDA receptor function in prefrontal cortex.Neurobiol Dis. 2016 Nov;95:194-203. doi: 10.1016/j.nbd.2016.07.024. Epub 2016 Jul 27. Neurobiol Dis. 2016. PMID: 27475724 Free PMC article.
References
-
- Aldridge JW, Berridge KC, Rosen AR. Basal ganglia neural mechanisms of natural movement sequences. Can J Physiol Pharmacol. 2004;82:732–739. - PubMed
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
-
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. - PubMed
-
- Bienvenu OJ, Wang Y, Shugart YY, Welch JM, Grados MA, Fyer AJ, Rauch SL, McCracken JT, Rasmussen SA, Murphy DL, Cullen B, Valle D, Hoehn-Saric R, Greenberg BD, Pinto A, Knowles JA, Piacentini J, Pauls DL, Liang KY, Willour VL, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:710–720. - PMC - PubMed
-
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163:121–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
